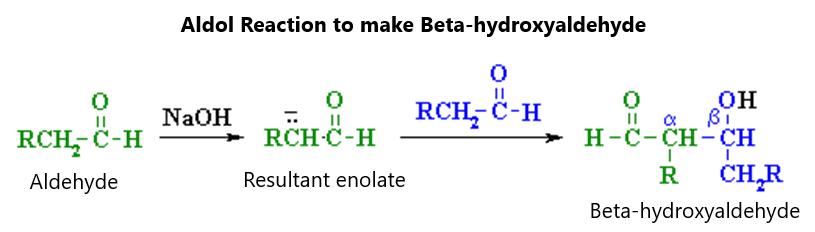
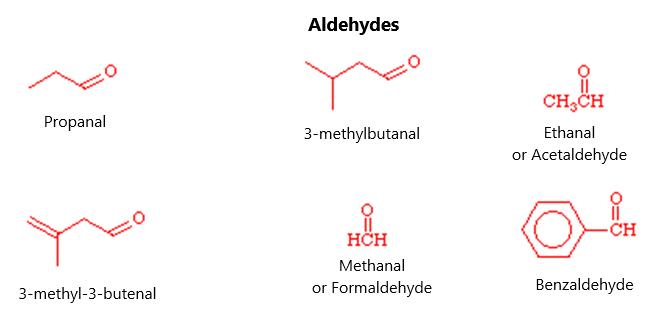
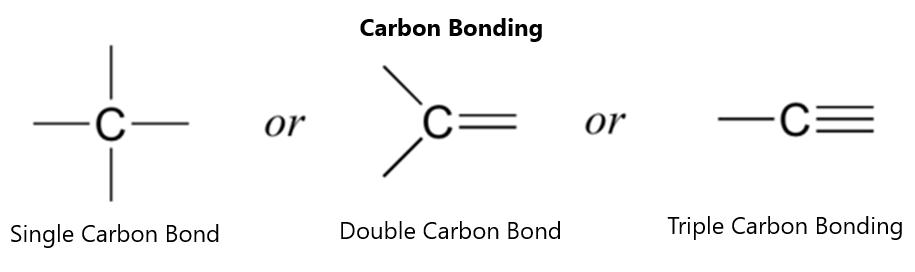
Figure 12.
ORGANIC MOLECULAR CHARGES You should also have an understanding of what it means to be a charged molecule. This is relatively easy to understand when it comes to ionized molecules like sodium, which becomes Na+, and chlorine, which becomes Cl- when ionized. What you need to know now is that organic molecules can also be charged. An example of this is methanol, which is CH3OH, which can be neutral. It can also lose an H+ ion to make CH3O- (an anion) or can gain a hydrogen ion to become CH3OH2+. In these cases, the positive charge or negative charge is on the oxygen atom. This can be further categorized as the “formal charge”, which is the charge specifically on the oxygen atom rather than on the molecule as a whole or on any other part of the molecule. To find the formal charge on different atoms of an organic molecule, it is necessary to add up the valence electrons. In the case of oxygen, an unbound oxygen atom has six valence electrons. When it is bound in the methanol molecule, there are two electrons used to make the bonds to carbon and hydrogen and four left over. These four electrons left over are all “owned” by the oxygen molecule. The bound electrons with hydrogen are “half-owned”. Taking the total valence electrons and subtracting the unbound electrons around the atom and half of the bound electrons, gives a net charge of 0 on a methanol molecule. Figure 13 demonstrates the formal charge of -1 on the oxygen atom in the methanol anion:
17