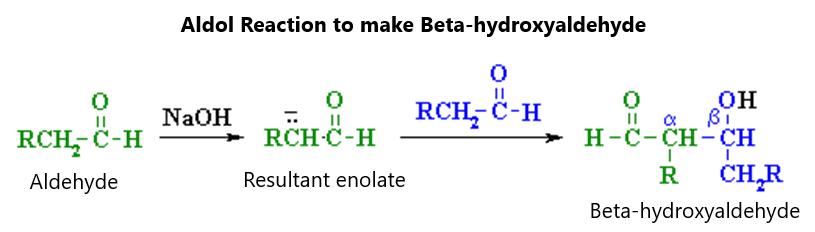
Figure 31.
The difference between diastereomers and enantiomers is that enantiomers differ in all of the stereocenters. This makes enantiomers mirror images of one another. Diastereomers will have different physical properties and different chemical properties. If there is a single bond involved, there can be rotation around the carbon atom so that the molecule is the same, regardless of the rotational status.
ENANTIOMER Enantiomers are mirror images that cannot be superimposed upon one another. It is the equivalent of the left hand and the right hand of a person. The thumb makes them not able to be directly superimposed. The mixture of two enantiomers is called a racemic mixture if they have the same concentration. There are S and R stereoisomers, which stand for “left” and “right”, respectively. Enantiomers are important in biochemistry because they act differently in biological systems. Drugs, especially, can be left-handed or right-handed. The general idea is that one handedness will have the primary pharmacological activity, while the other handedness will not be as pharmacologically active. Besides the R/S system, there can be a +/system and a d- and l- system. The D/L system stands for dextro and levo, respectively (right and left-handed). One example is the drug called thalidomide, which was a sedative. One of the enantiomers had sedative properties, while the equally prevalent enantiomer did not have sedative properties but caused birth defects.
40