I J S PT











LightForce® Therapy Lasers empower you to treat soft tissue with confidence. Harnessing photobiomodulation (PBM)—a powerful form of light therapy—our lasers stimulate cellular metabolism to help treat muscle and joint pain from acute and chronic conditions.
Equipped with smart features like dosing recommendations, real-time visual and haptic feedback, and convenient portability, our range of therapy lasers combines a fusion of power with intelligence to enhance the patient and user experience. With the ability to reach deep tissues, LightForce lasers can cut the time needed by clinicians to treat patients—making light work of pain.
TRUSTED GLOBALLY
More than 250 professional and collegiate sports teams around the world trust LightForce Therapy Lasers to provide rehabilitation and pain management.
Scan the QR code to request a demo, or visit https://learn.chattanoogarehab.com/ijspt-journal-2024.



Turner A Blackburn, APTA Life Member, AT-Ret, AOSSM-Ret President
Mary Wilkinson Executive Director
Michael Voight Executive Editor and Publisher
Joe Black, PT, DPT, SCS, ATC
Eric Fernandez
Jay Greenstein, DC
Skip Hunter, PT, ATC-Ret
Russ Paine, PT, DPT
Tim Tyler, PT, ATC
Sports Legacy Advisory Board
Turner A. Blackburn, PT, ATC
George Davies, PT, DPT, MEd, SCS, ATC, LAT, CSCS, PES, FAPTA
Terry Malone, PT, PhD
Bob Mangine, PT
Barb Sanders, PT, PhD
Tim Tyler, PT, ATC
Kevin Wilk, PT, DPT, FAPTA
Executive Editor/Publisher
Michael L. Voight, PT, DHSc, OCS, SCS, ATC, CSCS
Executive Director/Operations and Marketing
Mary Wilkinson
Editor in Chief
Barbara Hoogenboom, PT, EdD, SCS, ATC
Managing Editor
Ashley Campbell, PT, DPT, SCS, CSCS
Manuscript Coordinator
Casey Lewis, PTA, ATC
AMERICAN SPORTS MEDICINE INSTITUTE
Publisher
Contact Information
International Journal of Sports Physical Therapy 6011 Hillsboro Pike Nashville, TN 37215, US, http://www.ijspt.org
IJSPT is a monthly publication, with release dates on the first of each month.
ISSN 2159-2896
Underwriting Sponsor Genie Health
Founding Sponsors Enovis Exertools Hyperice Trazer Woodway
Platinum Sponsors ATI Elvation
Gold Sponsors Hawkgrips Kayezen Structure + Function Education Winback Partners
Northeast Seminars Academy of Human Movement
American Academy of Sports Physical Therapy
IJSPT is an official journal of the International Federation of Sports Physical Therapy (IFSPT). Countries with access to IJSPT as a member benefit. Reach us at www.ifspt.org.

IJSPT is an official journal of the ICCUS Society for Sports Rehabilitation. www.iccus.org






Stand out in your community with a diversified patient experience. Designed to improve outcomes, attract new patients, and increase revenue through insurance, cash-based services, and retail sales.


Gain access to a robust library of research, clinical education, and marketing tools including:
• On-demand clinical education courses
• Written treatment protocols
• Over 50 research studies specific to Hyperice technology
• Marketing tips and best practices including social media content, videos, and more
• Live trainings

Executive Editor/Publisher
Michael L. Voight, PT, DHSc, OCS, SCS, ATC, CSCS
Belmont University
Nashville, Tennessee – USA
Editor in Chief
Barbara Hoogenboom, PT, EdD, SCS, ATC
Grand Valley State University
Grand Rapids, Michigan - USA
Managing Editor
Ashley Campbell, PT, DPT, SCS, CSCS
Nashville Sports Medicine and Orthopaedic Center
Nashville, Tennessee – USA
Manuscript Coordinator
Casey Lewis, PTA, ATC
Nashville Sports Medicine and Orthopaedic Center
Nashville, Tennessee – USA
Executive Director/Marketing
Mary Wilkinson
Indianapolis, Indiana – USA
Editors
Robert Manske PT, DPT, Med, SCS, ATC, CSCS
University of Wichita Wichita, KS, USA
Terry Grindstaff, PT, PhD, ATC, SCS, CSCS
Creighton University Omaha, NE, USA
Phil Page PT, PhD, ATC, CSCS
Franciscan University DPT Program
Baton Rouge, LA, USA
Kevin Wilk PT, DPT, FAPTA
Clinical Viewpoint Editor Champion Sports Medicine Birmingham, AL, USA
International Editors
Luciana De Michelis Mendonça, PT, PhD UFVJM
Diamantina, Brazil
Colin Paterson PT, MSc PGCert(Ed), MCSP, RISPT, SFHEA
University of Brighton Brighton, England, UK
Chris Napier, PT, PhD
Clinical Assistant Professor
University of British Coumbia, Vancouver, BC, Canada
Nicola Phillips, OBE, PT, PhD, FCSP Professor School of Healthcare Sciences Cardiff University, Cardiff, Wales, UK
Associate Editors
Eva Ageberg, PT, PhD Professor, Lund University Lund, Sweden
Lindsay Becker, PT, DPT, SCS, USAW Buckeye Performance Golf Dublin, Ohio, USA
Keelan Enseki, PT, MS, OCS, SCS, ATC University of Pittsburgh Pittsburgh, PA, USA
John Heick, PT, PhD, DPT, OCS, NCS, SCS
Northern Arizona University Flagstaff, AZ, USA
Julie Sandell Jacobsen, MHSc, PhD
VIA University Aarhus, Denmark
RobRoy L. Martin, PhD, PT, CSCS
Duquesne University Pittsburgh, PA, USA
Andrea Mosler, PhD, FACP, FASMF
La Trobe Sport and Exercise Medicine Research Centre, School of Allied Health, Human Services and Sport, La Trobe University Melbourne, Victoria, Australia
Brandon Schmitt, DPT, ATC
PRO Sports Physical Therapy Scarsdale, NY, USA
Barry Shafer, PT, DPT
Elite Motion Physical Therapy Arcadia, CA, USA
Laurie Stickler, PT, DHSc, OCS
Grand Valley State University
Grand Rapids, MI, USA
Editorial Board
James Andrews, MD
Andrews Institute & Sports Medicine Center
Gulf Breeze, AL, USA
Amelia (Amy) Arundale, PT, PhD, DPT, SCS
Red Bull/Ichan School of Medicine
Salzburg, Austria/New York, NY, USA
Gary Austin, PT PhD
Belmont University Nashville, TN, USA
Roald Bahr, MD
Oslo Sports Trauma Research Center
Oslo, Norway
Lane Bailey, PT, PhD
Memorial Hermann IRONMAN Sports Medicine Institute
Houston, Texas, USA
Gül Baltaci, PT,Ph.D. Professor, CKTI, FACSM
Private Guven Hospital Ankara, Turkey
Asheesh Bedi, MD
University of Michigan
Ann Arbor, MI, USA
David Behm, PhD Memorial University of Newfoundland St. John's, Newfoundland, Canada
Barton N. Bishop, PT, DPT, SCS, CSCS Kaizo Clinical Research Institute Rockville, Maryland, USA
Mario Bizzini, PhD, PT Schulthess Clinic Human Performance Lab Zürich, Switzerland
Joe Black, PT, DPT, SCS, ATC Total Rehabilitation Maryville, Tennesse, USA
Turner A. "Tab" Blackburn, APTA Life Member, ATC-Ret, AOSSM-Ret NASMI Lanett, AL, USA
Lori Bolgla, PT, PhD, MAcc, ATC Augusta University Augusta, Georgia, USA
Matthew Briggs The Ohio State University Columbus, OH, USA
Tony Brosky, PT, DHSc, SCS Bellarmine University Louisville, KY, USA
Brian Busconi, MD UMass Memorial Hospital Boston, MA, USA
Robert J. Butler, PT, PhD St. Louis Cardinals St. Louis, MO, USA
Duane Button, PhD Memorial University St. Johns, Newfoundland, Canada
J. W. Thomas Byrd, MD Nashville Sports Medicine and Orthopaedic Center Nashville, TN, USA
Lyle Cain, MD Andrews Institute & Sports Medicine Center Birmingham, AL, USA
Gary Calabrese, PT, DPT Cleveland Clinic Cleveland, Ohio, USA
Meredith Chaput, PT, DPT, SCS Ohio University Athens, OH, USA
Rita Chorba, PT, DPT, MAT, SCS, ATC, CSCS United States Army Special Operations Command Fort Campbell, KY, USA
John Christoferreti, MD Texas Health Dallas, TX, USA
Richard Clark, PT, PhD Tennessee State University Nashville, TN, USA
Juan Colado, PT, PhD University of Valencia Valencia, Spain
Brian Cole, MD Midwest Orthopaedics at Rush Chicago, IL, USA
Ann Cools, PT, PhD
Ghent University Ghent, Belgium
Andrew Contreras, DPT, SCS Washington, DC, USA
George Davies, PT, DPT, MEd, SCS, ATC, LAT, CSCS, PES, FAPTA
Georgia Southern University Savannah, Georgia, USA
Pete Draovich, PT
Jacksonville Jaguars Footbal Jacksonvile, FL, USA
Jeffrey Dugas, MD Andrews Institute & Sports Medicine Center Birmingham, AL, USA
Jiri Dvorak, MD Schulthess Clinic Zurich, Switzerland
Todd Ellenbecker Rehab Plus Phoenix, AZ, USA
Carolyn Emery, PT, PhD University of Calgary Calgary, Alberta, Canada
Ernest Esteve Caupena, PT, PhD University of Girona Girona, Spain
Sue Falsone, PT, MS, SCS, ATC, CSCS, COMT Structure and Function Education and A.T. Still University Phoenix, Arizona, USA
J. Craig Garrison, PhD, PT, ATC, SCS Texas Health Sports Medicine Fort Worth, Texas, USA
Maggie Gebhardt, PT, DPT, OCS, FAAOMPT Fit Core Physical Therapy/Myopain Seminars Atlanta, GA and Bethesda, MD, USA
Lance Gill, ATC
LG Performance-TPI Oceanside, CA, USA
Phil Glasgow, PhD, MTh, MRes, MCSP Sports Institute of Northern Ireland Belfast, Northern Ireland, UK
Robert S. Gray, MS, AT Cleveland Clinic Sports Health Cleveland, Ohio, USA
Jay Greenstein, DC Kaizo Health Baltimore, MD, USA
Martin Hagglund, PT PhD
Linkoping University Linkoping, Sweden
Allen Hardin, PT, SCS, ATC, CSCS
University of Texas Austin, TX, USA
Richard Hawkins, MD
Professor of surgery, University of South Carolina
Adjunct Professor, Clemson University
Principal, Steadman Hawkins, Greenville and Denver (CU)
John D.Heick, PT, PhD, DPT, OCS, NCS, SCS
Northern Arizona University Flagstaff, AZ, USA
Tim Hewett, PhD
Hewett Consulting Minneapolis, Minnesota, USA
Per Hølmich, MD
Copenhagen University Hospital Copenhagen, Denmark
Kara Mae Hughes, PT, DPT, CSCS
Wolfe PT Nashville, TN, USA
Lasse Ishøi, PT, MSc
Sports Orthopedic Research Center
Copenhagen University Hospital Hvidovre, Denmark
Jon Karlsson, MD Sahlgrenska University Goteborg, Sweden
Brian Kelly, MD Hospital for Special Surgery New York, NY, USA
Benjamin R. Kivlan, PhD, PT, OCS, SCS Duquesne University Pittsburgh, PA, USA
Dave Kohlrieser, PT, DPT, SCS, OCS, CSCS
Ortho One Columbus, OH, USA
Andre Labbe PT, MOPT
Tulane Institute of Sports Medicine New Orleans, LA USA
Henning Langberg, PT, PhD University of Copenhagen Copenhagen, Denmark
Robert LaPrade, MD Twin Cities Orthopedics Edina, MN, USA
Lace Luedke, PT, DPT University of Wisconsin Oshkosh Oshkosh, WI, USA
Phillip Malloy, PT, PhD
Arcadia University/Rush University Medical Center Glenside, PA and Chicago, IL, USA
Terry Malone, PT, EdD, ATC, FAPTA University of Kentucky Lexington, KY, USA
Robert Mangine, PT University of Cincinnati Cincinnati, OH, USA
Eric McCarty, MD University of Colorado Boulder, CO, USA
Ryan P. McGovern, PhD, LAT, ATC Texas Health Sports Medicine Specialists Dallas/Fort Worth, Texas, USA
Mal McHugh, PhD
NISMAT
New York, NY, USA
Joseph Miller, PT, DSc, OCS, SCS, CSCS
Pikes Peak Community College Colorado Springs, CO, USA
Havard Moksnes, PT PhD
Oslo Sports Trauma Research Center Oslo, Norway
Andrew Murray, MD, PhD
European PGA Tour Edinburgh, Scotland, UK
Andrew Naylor, PT, DPT, SCS
Bellin Health
Green Bay, WI, USA
Stephen Nicholas, MD NISMAT New York New York, NY, USA
John O'Donnel, MD
Royal Melbourne Hospital Melbourne, Australia
Russ Paine, PT McGovern Medical School Houston, TX, USA
Snehal Patel, PT, MSPT, SCD
HSS Sports Rehabilitation Institute New York, NY, USA
Marc Philippon, MD
Steadman-Hawkins Clinic Vail, CO, USA
Kevin Plancher, MD, MPH, FAAOS
Plancher Orthopedics and Sports Medicine
New York, NY USA
Marisa Pontillo, PT, PhD, DPT, SCS
University of Pennsylvania Health System Philadelphia, PA, USA
Matthew Provencher, MD
Steadman Hawkins Clinic Vail, CO, USA
Charles E. Rainey, PT, DSc, DPT, MS, OCS, SCS, CSCS, FAAOMPT
United States Public Health Service Springfield, MO, USA
Alexandre Rambaud, PT PhD Saint-Etienne, France
Carlo Ramponi, PT Physiotherapist, Kinè Rehabilitation and Orthopaedic Center Treviso, Italy
Michael Reiman, PT, PhD Duke University Durham, NC, USA
Mark F. Reinking, PT, PhD, SCS, ATC Regis University Denver, CO, USA
Mark Ryan, ATC Steadman-Hawkins Clinic Vail, CO, USA
David Sachse, PT, DPT, OCS, SCS USAF San Antonio, TX, USA
Marc Safran, MD Stanford University Palo Alto, CA, USA
Alanna Salituro, PT, DPT, SCS, CSCS New York Mets Port Saint Lucie, FL, USA
Mina Samukawa, PT, PhD, AT (JSPO) Hokkaido University Sapporo, Japan
Barbara Sanders, PT, PhD, FAPTA, Board Certified Sports Physical Therapy Emeritus Professor and Chair, Department of Physical Therapy Texas State University Round Rock, TX, USA
Felix “Buddy” Savoie, MD, FAAOS Tulane Institute of Sport Medicine New Orleans, LA, USA
Teresa Schuemann, PT, DPT, ATC, CSCS, Board Certified Specialist in Sports Physical Therapy Evidence in Motion Fort Collins, CO, USA
Timothy Sell, PhD, PT, FACSM Atrium Health Musculoskeletal Institute Charlotte, NC, USA
Andreas Serner, PT PhD
Aspetar Orthopedic and Sports Medicine Hospital Doha, Qatar
Ellen Shanley, PT, PhD ATI Spartanburg, SC, USA
Karin Silbernagel, PT, PhD University of Delaware Newark, DE, USA
Holly Silvers, PT, PhD Velocity Physical Therapy Los Angeles, CA, USA
Lynn Snyder-Mackler, PT, ScD, FAPTA STAR University of Delaware Newark, DE, USA
Alston Stubbs, MD Wake Forest University Winston-Salem, NC, USA
Amir Takla, B.Phys, Mast.Physio (Manip), A/Prof
Australian Sports Physiotherapy The University of Melbourne Melbourne, Australia
Charles Thigpen, PhD, PT, ATC ATI
Spartanburg, SC, USA
Steven Tippett, PT, PhD, ATC, SCS Bradley University Peoria, IL, USA
Tim Tyler, PT, ATC NISMAT New York, NY, USA
Timothy Uhl, PT, PhD, ATC University of Kentucky Lexington, KY, USA
Bakare Ummukulthoum, PT University of the Witswatersrand Johannesburg, Gauteng, South Africa
Yuling Leo Wang, PT, PhD Sun Yat-sen University Guangzhou, China
Mark D. Weber, PT, PhD, SCS, ATC Texas Women’s University Dallas, TX, USA
Richard B. Westrick, PT, DPT, DSc, OCS, SCS US Army Research Institute Boston, MA, USA
Chris Wolfe, PT, DPT Belmont University Nashville, TN, USA
Tobias Wörner, PT, MSc Lund University Stockholm, Sweden
PAGE TITLE
ORIGINAL RESEARCH
834 Comparing Functional Movement Screen™ Scores Between Patients with Low Back Pain and Healthy Controls: A Systematic Review and Meta-Analysis. Alkhathami KM, Alqahtani B
849 Center of Pressure Velocity and Dynamic Postural Control Strategies Vary During Y-Balance and Star Excursion Balance Testing. Jagger KL, Harper B
856 Publicly Available Anatomic Total Shoulder Arthroplasty Rehabilitation Protocols Show High Variability and Frequent Divergence from the 2020 ASSET Recommendations. Mehta N, Acuna AJ, McCormick JR, et al.
868 Screening for Incidence and Effect of Pelvic Floor Dysfunction in College-Aged Athletes. Salvo CJ, Crewe A, Estes D, et al.
877 The Effects of TMR® Fab 6 on Hamstring Flexibility in Healthy Subjects; An Exploratory Observational Investigation. Patterson RD, Zettlemoyer A, Placzkowski M, et al.
CASE REPORT
888 Apprehension-Based Training: A Novel Treatment Concept for Anterior Shoulder Dislocation – A Case Report. Rabin A, Noyman L, Yaakobi N, et al.
898 Nonsurgical Management of Adductor-related groin pain with Ultrasound-Guided Platelet-Rich Plasma Injection and Physical Therapy in a Competitive Soccer Player: A Case Report. Zeppieri G, Smith MS, Roach RP.
CLINICAL COMMENTARY
910 A Rehabilitation Algorithm After Lateral Ankle Sprains in Professional Football (Soccer): An Approach Based on Clinical Practice Guidelines. Flore Z, Hambly K, De Coninck K, et al.
923 Enhancing Return to Alpine Skiing: Integrating Perceptual-Motor-Cognitive Considerations in Testing and Progressions: A Clinical Commentary. Smith C, Grooms DR, Bradley H.
MSK USB: TIPS AND TRICKS
935 Utilizing Diagnostic Musculoskeletal Ultrasound for Assessment of the Infraspinatus Muscle and Tendon: Implications for Rehabilitation Professionals. Manske RC, Voight M, Wolfe C, Page P.






Cohorts run for a duration of 13 months, August through the following September. Application windows opens from October-December of the preceding year through RF-PTCAS.










Most Advanced Electrotherapy Device: Powerful, intuitive and user-friendly
Treat up to three body zones at once on all types of tissues
Effective in less than 10 minutes Enter A New Era of Therapy
TECAR
HIGH FREQUENCY
Metabolic Action at Cell Level
Hi-TENS
LOW FREQUENCY IN PULSED HIGH FREQUENCY
Ultimate Pain Management
Hi-EMS
MEDIUM FREQUENCY
Deep Muscle Contraction



Alkhathami KM, Alqahtani B. Comparing the Scores of The Functional Movement ScreenTM in Individuals with Low Back Pain versus Healthy Individuals: A Systematic Review and Meta-Analysis. IJSPT. Published online July 1, 2024:834-848.
doi:10.26603/001c.120199
Khalid M. Alkhathami1 , Bijad Alqahtani1 a
1 Health Rehabilitation , Shaqra University
Keywords: low back pain, functional movement screen, pain, injury risk, systematic review https://doi.org/10.26603/001c.120199
International Journal of Sports Physical Therapy
Background
The Functional Movement Screen™ (FMS™) is widely used to assess functional movement patterns and illuminate movement dysfunctions that may have a role in injury risk. However, the association between FMS™ scores and LBP remains uncertain.
Objective
The purpose of this systematic review and meta-analysis was to examine functional movement scores among patients with low back pain (LBP) and healthy subjects with no LBP and review the validity of the FMS™ tool for screening functional movement among LBP patients.
Methods
The systematic review and meta-analysis included papers assessing functional movement among adult patients with LBP using the FMS™ through a literature review of five databases. The search strategy focused used relevant keywords: Functional movement screen AND low back pain. The review included all papers assessing functional movement among LBP adult patients (>18 years old) using the FMS™ published between 2003 to 2023. The risk of bias in the involved studies was evaluated using the updated Cochrane ROB 2 tool. Statistical analysis was conducted using Review Manager software, version 5.4. The meta-analysis included the total FMS™ score and the scores of the seven FMS™ movement patterns.
Results
Seven studies were included in this systematic review were considered to have low to unclear risk of bias. The meta-analysis revealed that the LBP group had a significantly lower total FMS™ score than the control group by 1.81 points (95% CI (-3.02, -0.59), p= 0.004). Patients with LBP had a significantly lower score than the control group regarding FMS™ movement patterns, the deep squat (p <0.01), the hurdle step (p <0.01), the inline lunge (P value <0.01), the active straight leg raise (p <0.01), the trunk stability push-up (p=0.02), and the rotational stability screens (p <0.01).
Lower scores on the FMS™ are associated with impaired functional movement. Identifying the specific functional movement impairments linked to LBP can assist in the creation of personalized treatment plans and interventions. Further research is needed to assess the association of cofounders, such as age, gender, and body mass index, with the FMS™ score among LBP patients and controls.
a
Corresponding author
Bijad Alqahtani
Department of Health Rehabilitation, College of Applied Medical Sciences at Shaqra, Shaqra University, Shaqra, 11961, Saudi Arabia Balqahtani@su.edu.sa https://orcid.org/0000-0001-7887-0979
Low back pain (LBP) is a widespread musculoskeletal condition that affects up to 80% of individuals throughout their lifetime.1 It is considered the most common disorder in gymnastics, football, volleyball, and tennis athletes, accounting for 20% of sports injuries involving the spine.2,3 LBP is typically categorized as mechanical, rheumatic, infectious, tumoral, or mental, with mechanical LBP being the most common, around 90% of cases.4 Various factors may contribute to LBP incidence, including age, smoking, genetics, weight (gain), improper weightlifting, nutritional disorders, decreased flexibility and hydration, acute injuries, chronic stress, and poor physical conditions.5,6
The evaluation of patients with LBP, including conducting functional evaluations, is crucial in the clinical field.7 Several tools are used to assess patients with LBP, such as the Back Pain Functional Score, Oswestry Disability Index (ODI), Numerical Rating Scale (NRS), Pain Self-Efficacy Questionnaire (PSEQ), Patient-specific Functional Scale (PSFS), and the Functional Movement Screen™ (FMS™).8
The FMS™ assesses movement patterns and identifies restrictions and compensations. The primary objective of the FMS™ is to evaluate an individual’s ability to perform various movements, including those related to flexibility, range of motion, muscle strength, coordination, balance, and proprioception. It consists of seven component movements; the deep squat, hurdle step, inline lunge, shoulder mobility, active straight leg raise, push-up, and rotational stability movements. Several of these movements are performed bilaterally and when tests are performed bilaterally, the lower of the two scores is used for analysis.The assessment is carried out through standardized verbal instructions and visual inspection. FMS™ scores are assigned based on task performance, including movement conditions with or without pain and symmetry 9,10 The score for each movement ranges from 0 to 3, with a total cumulative score ranging from 0 to 21 points.11,12 Lower scores (≤14) on the FMS™ indicate impaired functional movements associated with the potential for a higher risk of injury 13
The purpose of this systematic review and meta-analysis is to examine functional movement scores among patients with low back pain (LBP) and healthy subjects with no LBP and review the validity of the FMS™ tool for screening functional movement among LBP patients.
This systematic review complied with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria.14
The systematic review and meta-analysis were conducted through a thorough literature search of PubMed, Medline, Ovid, Scopus, and Central research databases using the keywords Functional movement screen AND low
back pain. Studies published from 2003 to 2023 were screened to select studies that matched the inclusion/ exclusion criteria. Furthermore, selected study references were reviewed manually to identify similar studies. Only studies that compared the FMS™ between patients with chronic LBP and healthy control subjects were incorporated in the meta-analysis.
Papers assessing functional movement among adult patients with LBP (>18 years old) with FMS™ and published from 2003 to 2023 were included. Studies published in languages other than English were excluded. Narrative reviews, systematic reviews, consensus reports, case reports, case series, duplicated studies, published before 2003, studies with insufficient data or findings regarding FMSTM score, studies with irrelevant findings, studies that used other functional movement tools or assessed patients with another type of pain, and studies for which full text was unavailable were also excluded. Only studies that compared the FMS™ between patients with chronic LBP and healthy control subjects were incorporated in the meta-analysis.
First, title and abstract screening was performed by the authors. Relevant full-text papers and evaluated the research for inclusion criteria were examined by one author After articles were selected for inclusion, data were extracted and entered in a Microsoft Excel spreadsheet. Extracted data included authors, year of publication, objective, study design, sample size, gender, age, intervention, assessment tool, results, and outcome. Further data for the meta-analyses included total FMS™ score, in addition to scores of the seven FMS™ composite tests were extracted from the articles included in this systematic review.
The risk of bias in the incorporated papers was evaluated using the Cochrane Risk of Bias 2 (ROB 2) tool. The ROB 2 tool offers a structured, standardized, and flexible approach to assessing the risk of bias in randomized trials and nonrandomized studies of interventions.15 The tool assesses quality based on five major domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. Each domain has a set of signaling questions that inform the risk of bias judgment for that domain. Based on the responses to each domain, the options for a domain-level risk-of-bias judgment are ‘Low’, ‘High’, or ‘Unclear’ risk of bias. A total or overall risk of bias score for each article was not determined.
Review Manager, version 5.4 (The Cochrane Collaboration, Oxford, England) was used for data entry and analysis. The standard deviation (SD) of the means were estimated from CI limits or standard mean difference (if not provided). The size of the continuous outcomes effect was reported as standard mean difference (SMD), and the precision of effect size was also reported as a 95% confidence interval (CI). DerSimonian and Laird’s random-effects model was used to compute SMD.16 Cochrane Q tests and Leave one out (I²) statistics were used to evaluate the heterogeneity and inconsistency across the studies. Leave one out metaanalysis was used for sensitivity analysis to recognize that the overall effect (against which heterogeneity is measured) changes each time an influential study is excluded.17 Statistical significance was set at p < 0.01 for Cochrane Q tests. If a high heterogeneity was detected, a leave-one-out test (removing studies one by one) was performed.
The initial search strategy provided 91 papers, of which 12 were omitted as duplicates. Regarding the remaining 79 articles, 34 were excluded because they did not match the inclusion criteria. Following screening and assessment, 38 additional articles were excluded because they did not match the study’s objective. Seven studies were considered suitable for and included in this systematic review (Figure 1).
In terms of the seven included papers, all were published between 2016 and 2023 (Table 1). The articles included 272 adult subjects with an age range of 18-65 years old. The study subjects were patients with LBP, and either athletes, or healthy controls without LBP. The study design varied among the articles; one study was a double-blinded randomized clinical trial,18 one was a reliability and validity study,19 one was a cross-sectional study,20 and Four were prospective studies.7,21‑23 Some studies included either males or females, and others included both genders. All included studies assessed LBP using the FMSTM , but one study also used the Numeric Pain Rating Scale (NPRS) and Oswestry Low Back Pain Disability Questionnaire (OSW).18 Only one study used intervention which included spinal stabilization exercises (SSEs) and general exercises (GEs).18
Table 2 shows a representation of the risk of bias assessment.
Regarding sequence generation and allocation concealment, six studies had a low risk of bias and an unclear risk of bias. In blinding of participants and personnel and blinding of outcome assessment, two studies had a high risk of bias, one study had a low risk of bias, and four studies had an unclear risk of bias. Moreover, five studies showed an unclear risk of bias, and two studies had a high risk of bias
regarding the incomplete outcome data section. All studies had a high risk of bias in the selective reporting section. However, regarding other sources of bias, five studies had a high risk of bias, while two had an unclear risk of bias. Overall, the included studies should be considered to have low to unclear risk of bias.
FMS TOTAL SCORE AMONG LBP PATIENTS AND CONTROL GROUP
The total score of FMS™ among LBP patients and the control group was available in three papers (144 patients).7,19, 21 The analysis revealed that the LBP group had a significantly lower total FMS™ score than the control group by 1.81 (95% CI (-3.02, -0.59), p=0.004). In addition, a significantly high heterogeneity was found (I2= 89%, p<0.001).
Three studies7,19,21 (144 patients) reported the scores of the seven FMS™ movement patterns between the patients with LBP and the control group. Of note, when tests are performed bilaterally (hurdle step, in line lunge, shoulder mobility, active straight leg raise, trunk stability push up, and rotary stability), the lower of the two scores is used for analysis, resulting in a single score for those tests.
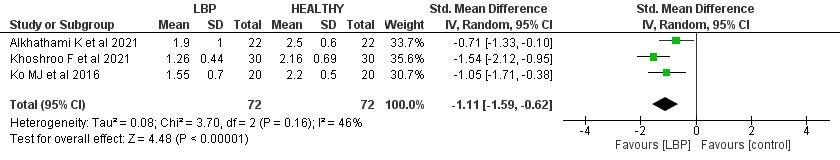
There was a significant difference between the patients with LBP control group scores with SMD -1.11 (95% CI (-1.59, -0.62), p< 0.00). Low heterogeneity was found (I2= 46%, p= 0.16).
The hurdle step mean score was significantly lower in the LBP group when compared to the control group by 1.41 (95% CI (-2.01, -0.81), p< 0.001). High heterogeneity was found (I2= 85%, p<0.001). A leave-one-out test was done, the Alhathaml et al. study was removed, and the heterogeneity became (I2= 45%, p= 0.18).
Regarding the inline lunge score, the patients with LBP had significantly lower scores than the control group, with SMD -0.41 (95% CI (- 0.74, -0.08), p=0.02). No heterogeneity was found (I2= 0%, p= 0.54).
There was a non-significant difference in the shoulder mobility score among LBP patients and the control group, with SMD -0.39 (95% CI (-1.03, 0.25), p=0.23). Significant heterogeneity was found (I2= 73%, p-value= 0.03). A leaveone-out test was done, the Khohroo et al. study was removed, and the heterogeneity became (I2= 0%, p=0.77), and SMD became -0.06 (95% CI (-0.49, 0.37), p= 0.78).
Author, year Objective
Alkhathami K et al., 202318 Assessing SSEs effects on the level of movement performance, pain intensity, and disability among adults with CLBP. Doubleblinded randomized clinical trial.
Alkhathami K et al., 202119 It detects the reliability and validity of the FMS™ with a modified scoring system among young adults with and without LBP.
and validity study
SSEs vs. GEs FMS TM , NPRS, and OSW
Over eight weeks, there was a substantial difference in modified FMS TM scores between the SSE and GE groups. -The modified FMS TM scores of all patients improved significantly between two adjacent time points: from baseline to two weeks (p = 0.011), two weeks to four weeks (p = 0.001), and four weeks to eight weeks (p = 0.008).
The LBP group scored significantly lower than those without LBP (p-value = 0.008).
The modified FMSTM with a scoring system might effectively assess mobility quality in individuals with LBP.
-It is considered that the FMS TM can differentiate between people who have and do not have LBP. For doctors, FMS TM might be a helpful test for evaluating movement
Author, year
Khoshroo F et al., 202121
Comparing females with LBP functional movement patterns with NPDs.
Subjects with LBP and NPDs.
Enoki S et al., 202020
-Assessing and examining the physical characteristics of pole vaulters with chronic LBP
-Clarifying the association between FMS™ A crosssectional study
collegiate pole vaulters
± 2.22 and NPDs: 26.53 ±2.37.
FMS™ - Significant lower scores in LBPDs compared to NPDs in the FMS TM composite score (12.06 vs. 16.43, pvalue < 0.001). -There was a negative association between FMS TM composite score and LBP intensity (r (60) = –0.724, p < 0.001) and positive with LBP onset (r (60) = 0.277, p = 0.032) during prolonged standing.
-In the chronic LBP group, the difference between the passive and active SLR angle (SLR) was substantially greater than in the non-
Outcome quality and identifying mobility limits in LBP patients.
-LBPD females, who are at higher risk for developing LBP, had significantly lower functional movement quality patterns compared to NPDs.
-The FMS TM could predict subjects at risk for LBP development during prolonged standing.
- The CLPB group was far more likely to have an FMSTM composite score ≤ 14. -It is critical to examine the active straight leg rise (vs.
Author, year Objective
performance with and without chronic LBP chronic LBP group (p-value 0.05). -Those with persistent LBP were more likely to have an FMS TM 14 score. passive only) and basic motions of pole vaulters using the FMS TM .
SL et al., 201822
Assessing if the FMS™ and impairments can identify rowers at risk for LBP development.
Clay H et al., 20123 They were determining whether the FMS™ scores predict the incidence of all injuries, such as LBP, among female
There were no differences in FMS™ or impairments between the Uninjured and LBP groups. The FMS™ cutoff score was 16 points.
An FMS TM score of 16 predicted a small increased risk of LBP development (1.4) compared to individuals with scores over 16. However, the FMS TM is not suggested for screening female rowers since the risk ratio was minimal and the 95% confidence interval was broad.
-Subjects detected as a high risk of injury by the FMS™ were more likely to have LBP during the season (p-The FMS™ has been estimated to predict injury among athletes. -The FMS™ has indicated a higher
Author, year
Outcome collegiate rowers during one rowing season. value =0.036). -Individuals with LBP history were six times more likely to suffer from LBP during the season (pvalue=0.027). likelihood of a subjective report of LBP
Ko MJ et al., 20167 Comparing the FMS TM scores between CLBP patients and healthy control subjects with using the FMS™ as an evaluation tool for examining functional deficits of CLBP in patients.
FMS™ - CLBP patients scored significantly lower on total composite scores (10.95 ± 2.2 points) compared with the control group (14.40 ± 1.8 points), p<0.001). -LBP patients had significantly lower scores on deep squat (1.55 ± 0.7 vs. 2.20 ± 0.5 points, p=0.002), hurdle step (1.95 ± 0.4 vs. 2.45 ± 0.5 points, p=0.002), ASLR (1.85 ± 0.7 vs. 2.55 ± 0.8 points, p=0.005), and rotary stability (1.15 ± 0.4 vs.
The deep squat, hurdle step, active straight leg raise, and rotary stability tasks of FMS™ could be recommended as functional assessment tools to assess functional deficits in CLBP patients.
Author,
1.80 ± 0.4 points, p<0.001). -There were no significant differences between CLBP patients and the control group in inline lunge (1.90 ± 0.7 vs. 2.25 ± 0.7 points, pvalue= 0.133), shoulder mobility (1.75 ± 0.9 vs. 1.85 ± 0.6 points, pvalue= 0.811), and trunk stability pushup (0.95 ± 0.5 vs. 1.30 ± 0.6 points, pvalue=0.056).
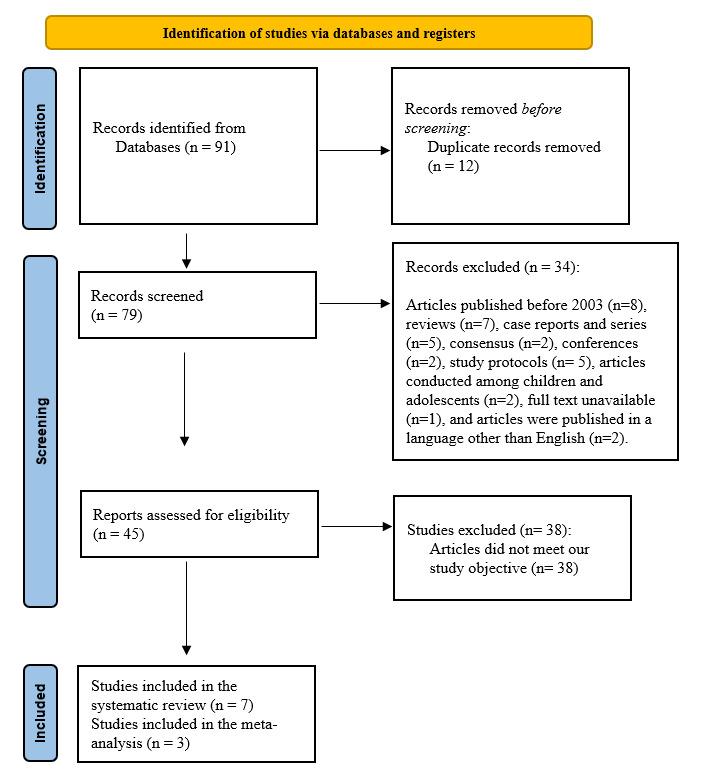
Figure 1. Studies involved in this systematic review
ACTIVE STRAIGHT-LEG RAISE SCORES
There was a significant difference between the LBP group and the controls, by SMD -0.75 (95% CI (-1.09, -0.41), p< 0.00). Furthermore, no heterogeneity was found (I2= 0%, p= 0.81).
STABILITY PUSH-UP SCORE
The LBP patients reported a significantly lower score in trunk stability push-up screening than the control by SMD -1.05 (95% CI (-1.88, -0.21), p=0.01). A significant high heterogeneity was found (I2= 81%, p=0.00). A leave-one-out test was done, the Khohroo et al. study was removed, and the heterogeneity became (I2= 0%, p=1.0), and SMD became -0.62 (95% CI (-1.06, -0.18), p= 0.00).
ROTATORY STABILITY SCORE
Regarding rotatory stability, a significant difference was revealed between the scores of the patients with LBP and the control group with SMD -0.82 (95% CI (-1.56, -0.08), p=0.03). Significant heterogeneity was found (I2= 78%, p=0.01). A leave-one-out test was done, the Ko et al. study
was removed, and the heterogeneity became (I2= 53%, p=0.14), and SMD became -0.48 (95% CI (-1.06, 0.09), p= 0.1).
Functional movement proficiency and examining movement patterns could demonstrate the foundation for lifelong physical activity While the FMS™ is considered a fundamental screening tool for assessing functional movement, previous research has been primarily focused on the application of FMS™ among athletes.24 This is the first systematic review and meta-analysis using the FMS™ to compare the functional movement scores among adult patients with LBP and healthy subjects. In addition, this study reviews the validity of the FMS™ tool for screening the functional movement abilities of LBP patients.
The FMS™ is a commonly utilized screening tool for evaluating functional movement, supported by experimental research conducted and synthesized to date. This research
Table 2. Risk-of-bias summary
Reference
Alkhathami
K et al., 2023,18
Alkhathami
K et al., 202119
Khoshroo
F et al., 202120
Enoki S et al., 202021
Gonzalez
SL et al., 201822
Clay H et al., 201623
Ko MJ et al., 20167
(+) Low risk of bias, (-) High risk of bias, (?) Unclear risk of bias
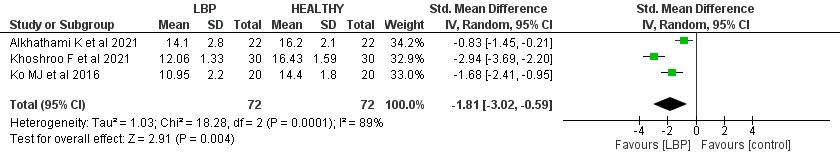
CI confidence interval, SD standard deviation, LBP low back pain.
encompasses diverse populations, including youth athletes and adults of both sexes.25,26
According to the screened studies in this systematic review, the mean total score of the FMS™ among LBP patients ranged from 10.95 to 14.1.7,19,21 The mean FMS™ scores for control groups ranged from 14.40 to 16.2. The meta-analysis found that LBP patients had a significantly lower total FMS™ score than the control group by 1.81 (p-value= 0.004). These findings support the literature and
suggest that LBP patients generally exhibit lower functional movement capabilities than individuals without LBP, as evidenced by their lower FMS™ scores, supporting the validity of the screening tool. These lower scores are due to these FMSTM tasks being accompanied by lower or upper extremity movement, and some patients with LBP have difficulty in properly recruiting certain muscles, such as trunk stability muscles, and often display limited hip joint mobility. This could be reflected in lower scores seen in those with
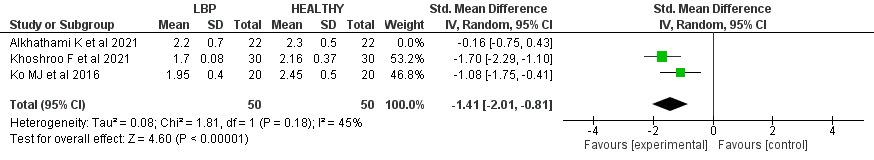
Figure 3. Forest plot of hurdle steps score and 95% CIs, grouped by LBP and control group.
CI confidence interval, SD standard deviation, LBP low back pain.

Figure 4. Forest plot of inline lunges score and 95% CIs, grouped by LBP and control group.
CI confidence interval, SD standard deviation, LBP low back pain.

Figure 5. Forest plot of shoulder mobility score and 95% CIs, grouped by LBP and control group.
CI confidence interval, SD standard deviation, LBP low back pain.
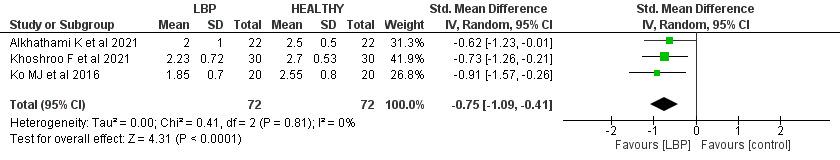
Figure 6. Forest plot of active straight-leg raises score and 95% CIs, grouped by LBP and control group.
CI confidence interval, SD standard deviation, LBP low back pain.
LBP on the deep squat, hurdle step, ASLR, and rotary stability movements.
INDIVIDUAL FMS™ MOVEMENT PATTERNS SCORES
LBP patients often demonstrate limited range of hip mobility, which may induce compensation in the lumbopelvic region during lower limb movement. According to obser-
vations, individuals with and without LBP showed unique movement patterns during forward bending.27 This observation further supports the existence of a biomechanical correlation between low back disorders and the functioning of other joints during dynamic tasks. Activities involving manual material handling and lifting have been linked to LBP 28 Among the techniques associated with these activities is the squat technique, which involves lifting with
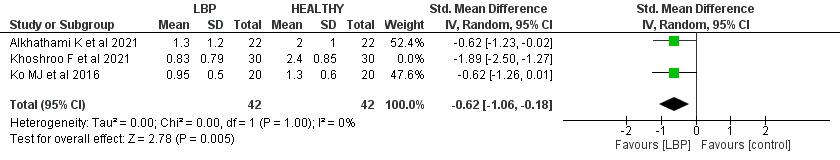
Figure 7. Forest plot of push-up score and 95% CIs, grouped by LBP and control group.
CI confidence interval, SD standard deviation, LBP low back pain.
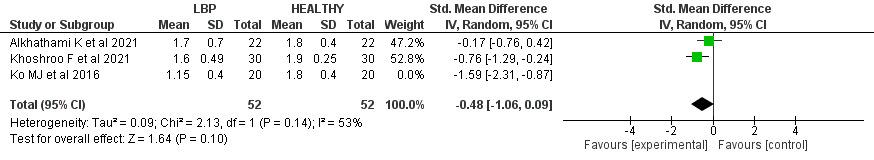
Figure 8. Forest plot of rotatory stability score and 95% CIs, grouped by LBP and control group.
CI confidence interval, SD standard deviation, LBP low back pain.
flexed knees.29 Squatting is fundamental to routine activities such as sitting down and standing up.30
In the present meta-analysis, there was a profound difference between the LBP patients and the control group by -1.11 regarding the deep squat score in the FMS™ (p< 0.00). Furthermore, the scores of the LBP patients regarding the deep squat movements ranged from 1.26 to 1.9 out of 3, which was lower than the control group, which scored from 2.16 to 2.5 out of 3. Accordingly, these findings indicate that the FMS™ can detect the deficiency in the deep squat movement among LBP patients.
The hurdle step requires appropriate stability and coordination between the hips and torso during the stepping motion. It was revealed that individuals with CLBP would demonstrate deficiencies in this movement pattern.9
The meta-analysis results found that the mean score of hurdle steps in the FMS™ was significantly lower in the LBP group compared to the control group by 1.41 (p < 0.001). Moreover, the hurdle step scores among patients with LBP had lower scores (mostly less than 2 points) ranging from 1.7, 1.95, and 2.45 out of 3 points. In comparison, the scores among control individuals ranged from 2.16, 2.3, and 2.45 (more than 2 points) out of 3 points.
The low hurdle step scores that LBP patients received highlight how restricted hip and spine mobility which may occur in LBP patients may affect this movement. In addition, these findings reveal that FMS™ is an appropriate mechanism to assess the hurdle step among LBP patients.
The inline lunge test requires ankle, knee, and hip stability in the stepping leg and controlled closed kinetic chain hip flexion. Additionally, mobility is required in hip abduction, ankle dorsiflexion, and rectus femoris flexibility of the stepping leg.
Poor performance in this test can be caused due to various factors. First, there may not be enough hip mobility in the stance or step leg. Second, the knee or ankle stability in the stance leg may be insufficient while performing the lunge. Last, in one or both hips, an imbalance between relative adductor weakness and abductor tightness OR abductor weakness and adductor tightness might contribute to poor test performance.9
The meta-analysis of the incorporated papers found that the inline lunge score in FMS™ among patients with LBP patients was significantly lower than the control group by 0.41 (p=0.02). Despite the statistical difference between the two groups, the value of the difference in score is less than 1 which clinically could be very minute. Moreover, the scores of patients with LBP on the inline lunge were somewhat similar to the control subjects’ scores (1.83 to 2.5 versus 2.1 to 2.6, respectively).
According to the included studies, there was no significant difference in the score of shoulder mobility among patients with LBP and the control group by -0.06 (p=0.78). However, the negative results mean the mean score of patients with LBP is lower than control group, no significant difference was found. Furthermore the included studies, patients with LBP scored similar or lower in the shoulder mobility movement than the control group.
The straight leg raise test is widely used to assess the active hamstring and gastro-soleus flexibility while preserving stability in the torso.31 Sciatica is discomfort that radiates from the buttocks to the legs and is commonly associated with LBP.32 LBP is among the most common indications for the use of the straight leg raise test.33
According to the present findings, the patients with LBP reported a score ranging from 1.85 to 2.23 out of 3, while the control group scored 2.5 to 2.77 out of 3. Moreover, there was a significant difference between the LBP group and the control one, by SMD -0.75, favoring healthy control cases (p< 0.00). However, the difference between the two groups is very low, which clinically could be very minute and may not impact the ability to distinguish adults with LBP from those without LBP
The push-up movement test is commonly used to investigate upper-limb muscular fitness, especially among young people.33.34 Low fitness in the trunk stability push-up test correlates with low back dysfunction and pain among middle-aged individuals.34
In this meta-analysis, the patients with LBP had lower push-up screening scores than the control scores by 1.05 (p=0.01). In the included studies, the LBP patients reported relatively low scores in the push-up screening, ranging from 0.83 to 1.3 out of 3. Low fitness in the modified pushup test has been associated with poor perceived health, low back dysfunction, and pain among middle-aged subjects. Also, poor endurance in the back musculature has been reported to be a risk factor for LBP 35
The rotatory stability test is performed with either lower or upper extremity movement. Shoulder flexion stimulates anterior displacement of the center of mass, placing greater demands on the trunk muscles to keep the center of mass over the base of support. Thus, trunk stability is required to sustain a neutral position. It was revealed that LBP patients have burdens that require proper recruitment of the trunk stability muscles before moving the limbs.5 Thus, compensation may occur among LBP patients during rotary stability tests due to inappropriate recruitment of the trunk stability muscles. This may lead to lower scores among LBP patients compared to healthy individuals.35‑37
In this meta-analysis, there was a significant difference between the scores of the patients with LBP and the control groups by 0.82 (p-value= 0.03). However, this difference between the two groups is less than 1, which clinically could be very minute.
This systematic review is limited to the few included studies that compare FMS™ among patients with LBP control groups. Furthermore, the review primarily focuses on adult subjects, and the generalizability of the findings to other populations, such as highly competitive and youth athletes, may be limited. Additionally, the review does not consider potential confounding factors such as pain or the influence of specific interventions or treatments on FMS™ scores. Further research is needed to assess the association of cofounders, such as age, gender, and body mass index, with the FMS™ score among LBP patients and the control group.
Low to unclear risk of bias studies included in this systematic review and meta-analysis provide valuable insights for clinicians and healthcare professionals while evaluating and treating patients with LBP. Lower scores on the FMS™ tool are associated with impaired functional movement and increased injury risk among LBP patients. Further well-designed research may be more specific in the targeted population and include FMS™ in LBP within one of its various subcategories, such as acute, chronic, and non-specific cases.
The authors would like to thank the Deanship of Scientific Research at Shaqra University for supporting this work.
All authors report no conflicts of interest.
Submitted: December 12, 2023 CDT, Accepted: May 04, 2024 CDT
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Walker BF. The prevalence of low back pain: A systematic review of the literature from 1966 to 1998. J Spinal Disord. 2000;13(3):205-217. doi:10.1097/ 00002517-200006000-00003
2. Andrews JR, Harrelson GL, Wilk KE. Physical Rehabilitation of the Injured Athlete: Expert ConsultOnline and Print Elsevier Health Sci; 2012.
3. Gur H. Epidemiology of pediatric sports injuries: Individual sports. J Sports Sci Med. 2005;4(2):214. doi:10.1159/000085330
4. Kiani Dehkordi K, Ebrahim K, Frastic S. Effective treatment of stretch step to keep changes in the face of resistance and liberation of the hip joint in patients with chronic low back pain. J Mov Sci Sport 2008;2(12):11-22.
5. Lee GK, Chronister J, Bishop M. The effects of psychosocial factors on quality of life among individuals with chronic pain. Rehab Couns Bull 2008;51(3):177-189. doi:10.1177/0034355207311318
6. Hodges PW Core stability exercise in chronic low back pain. Orthop Clin North Am 2003;34(2):245-254. doi:10.1016/s0030-5898(03)00003-8
7 Ko MJ, Noh KH, Kang MH, Oh JS. Differences in performance on the functional movement screen between chronic low back pain patients and healthy control subjects. J Phys Ther Sci 2016;28(7):2094-2096. doi:10.1589/jpts.28.2094
8. Koç M, Bayar B, Bayar K. A comparison of back pain functional scale with Roland Morris disability questionnaire, Oswestry disability index, and short form 36-health survey. Spine. 2018;43(12):877-882. doi:10.1097/BRS.0000000000002431
9. Cook G, Burton L, Hoogenboom B. Preparticipation screening: The use of fundamental movements as an assessment of function - part 1. N Am J Sports Phys Ther 2006;1(2):62-72.
10. Cook G, Burton L, Hoogenboom B. Preparticipation screening: the use of fundamental movements as an assessment of function - part 2. N Am J Sports Phys Ther. 2006;1(3):132-139.
11. Cook G, Burton L, Hoogenboom BJ, Voight M. Functional movement screening: The use of fundamental movements as an assessment of function - part 1. Int J Sports Phys Ther. 2014;9(3):396-409.
12. Cook G, Burton L, Hoogenboom BJ, Voight M. Functional movement screening: The use of fundamental movements as an assessment of function-part 2. Int J Sports Phys Ther 2014;9(4):549-563.
13. Kiesel K, Plisky P, Butler R. Functional movement test scores improve following a standardized offseason intervention program in professional football players. Scand J Med Sci Sports. 2011;21(2):287-292. doi:10.1111/j.1600-0838.2009.01038.x
14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. doi:10.1016/j.ijsu.2021.105906
15. Sterne JA, Hernán MA, Reeves BC, et al. ROBINSI: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. doi:10.1136/bmj.i4919
16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177-188. doi:10.1016/0197-2456(86)90046-2
17. Higgins JP. Commentary: Heterogeneity in metaanalysis should be expected and appropriately quantified. Int J Epidemiol 2008;37(5):1158-1160. doi:10.1093/ije/dyn204
18. Alkhathami K, Alshehre Y, Brizzolara K, Weber M, Wang-Price S. Effectiveness of spinal stabilization exercises on movement performance in adults with chronic low back pain. Int J Sports Phys Ther 2023;18(1):169-172. doi:10.26603/001c.68024
19. Alshehre Y, Alkhathami K, Brizzolara K, Weber M, Wang-Price S. Reliability and validity of the Ybalance test in young adults with chronic low back pain. Int J Sports Phys Ther. 2021;16(3):628-635. doi:10.26603/001c.23430
20. Enoki S, Kuramochi R, Murata Y, Tokutake G, Shimizu T. The relationship between chronic low back pain and physical factors in collegiate pole vaulters: A cross-sectional study Int J Sports Phys Ther. 2020;15(4):537-547. doi:10.26603/ijspt20200537
21. Khoshroo F, Seidi F, Rajabi R, Thomas A. A comparison of functional movement patterns between female low back pain developers and nonpain developers. Work 2021;69(4):1247-1254. doi:10.3233/WOR-213545
22. Gonzalez SL, Diaz AM, Plummer HA, Michener LA. Musculoskeletal screening to identify female collegiate rowers at risk for low back pain. J Athl Train. 2018;53(12):1173-1180. doi:10.4085/ 1062-6050-50-17
23. Clay H, Mansell J, Tierney R. Association between rowing injuries and the functional movement screenTM in female collegiate division I rowers. Int J Sports Phys Ther 2016;11(3):345-349.
24. O’Brien W, Khodaverdi Z, Bolger L, Tarantino G, Philpott C, Neville RD The Assessment of functional movement in children and adolescents: A systematic review and meta-analysis. Sports Med 2021;52(1):37-53. doi:10.1007/s40279-021-01529-3
25. Perry FT, Koehle MS. Normative data for the functional movement screen in middle-aged adults. J Strength Cond Res 2013;27(2):458-462. doi:10.1519/ JSC.0b013e3182576fa6
26. Schneiders AG, Davidsson A, Hörman E, Sullivan SJ. Functional movement screen normative values in a young, active population. Int J Sports Phys Ther. 2011;6(2):75-82.
27 Kim MH, Yi CH, Kwon OY, et al. Comparison of lumbopelvic rhythm and flexion-relaxation response between 2 different low back pain subtypes. Spine 2013;38(15):1260-1267 doi:10.1097/ BRS.0b013e318291b502
28. Marras WS, Lavender SA, Leurgans SE, et al. The role of dynamic three-dimensional trunk motion in occupationally-related low back disorders. The effects of workplace factors, trunk position, and trunk motion characteristics on risk of injury Spine 1993;18(5):617-628. doi:10.1097/ 00007632-199304000-00015
29. Mirakhorlo M, Azghani MR. Similarity of different lifting techniques in trunk muscular synergies. Acta Bioeng Biomech 2015;17(4):21-29.
30. Czaprowski D, Biernat R, Kędra A. Squat - Rules of performing and most common mistakes. Pol J Sport Tour 2012;19(1):3-7 doi:10.2478/v10197-012-0001-6
31. Pesonen J, Shacklock M, Rantanen P, et al. Extending the straight leg raise test for improved clinical evaluation of sciatica: Reliability of hip internal rotation or ankle dorsiflexion. BMC Musculoskelet Disord. 2021;22(1):303. doi:10.1186/ s12891-021-04159-y
32. Hill JC, Konstantinou K, Egbewale BE, Dunn KM, Lewis M, van der Windt D. Clinical outcomes among low back pain consulters with referred leg pain in primary care. Spine 2011;36(25):2168-2175. doi:10.1097/BRS.0b013e31820712bb
33. Catley MJ, Tomkinson GR. Normative healthrelated fitness values for children: Analysis of 85347 test results on 9-17-year-old Australians since 1985. Br J Sports Med 2013;47(2):98-108. doi:10.1136/ bjsports-2011-090218
34. Suni JH, Oja P, Miilunpalo SI, Pasanen ME, Vuori IM, Bös K. Health-related fitness test battery for adults: Associations with perceived health, mobility, and back function and symptoms. Arch Phys Med Rehabil 1998;79(5):559-569. doi:10.1016/ s0003-9993(98)90073-9
35. Kang MH, Jang JH, Kim TH, Oh JS. Effects of shoulder flexion loaded by an elastic tubing band on EMG activity of the gluteal muscles during squat exercises. J Phys Ther Sci. 2014;26(11):1787-1789. doi:10.1589/jpts.26.1787
36. Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil 1999;80(9):1005-1012. doi:10.1016/ s0003-9993(99)90052-7
37 Lee SH, Kim TH, Lee BH. The effect of abdominal bracing in combination with low extremity movements on changes in thickness of abdominal muscles and lumbar strength for low back pain. J Phys Ther Sci 2014;26(1):157-160. doi:10.1589/jpts.26.157

Jagger KL, Harper B. Center of Pressure Velocity and Dynamic Postural Control Strategies Vary During Y-Balance and Star Excursion Balance Testing. IJSPT. Published online July 1, 2024:849-855. doi:10.26603/001c.118943
Kristen
L Jagger1 a , Brent Harper2
1 School of Physical Therapy, Regis University, 2 Physical Therapy, Chapman University
Keywords: postural balance, y-balance test lower quarter, star excursion balance test
https://doi.org/10.26603/001c.118943
Background
Dynamic postural control (DPC) describes an individual’s ability to maintain balance within their base of support in both anticipatory and reactive balance situations and has been measured using center of pressure (COP) velocity. Common standardized DPC assessments for active adults include the modified Star Excursion Balance Test (MSEBT) and the Y-Balance Test (YBT).
Hypothesis/Purpose
The purpose of this study was to explore DPC during performance of the MSEBT, the YBT, and a modified version of the YBT, the MYBT It was hypothesized that feedback from the YBT/MYBT reach indicator would enhance DPC.
Study Design
Cross-sectional study
Methods
Twenty-one participants (9 females, 12 males, mean age 24.5±1.2 years) performed three trials in each direction (anterior-AN, posteromedial-PM, and posterolateral-PL) on each balance test during one session. The YBT frame was placed atop a force plate for all testing. Frontal and sagittal plane COP velocities (COPx and COPy, respectively) were recorded throughout each trial and resultant COP (COPr) velocities were calculated.
Results
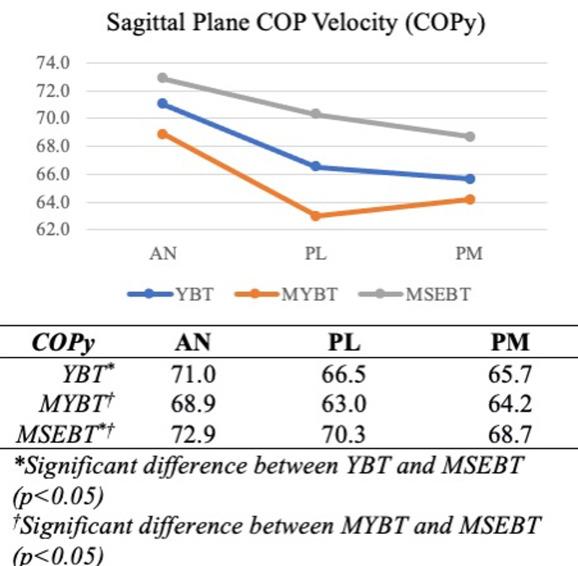
Significant main effects were present for test (F=4.485, p<0.001) and reach direction (F=61.594, p<0.001). Post hoc analyses for test indicated significant differences in COPy between YBT and MSEBT (p=0.034) and between MYBT and MSEBT (p<0.001), as well as significant differences in COPr between MYBT and MSEBT (p=0.002). Post hoc analyses for reach direction revealed significant differences in COPx between AN and both PM (p<0.001) and PL (p<0.001) directions, in COPy between AN and PM (p<0.001) and PL (p<0.001) directions, and COPr between AN and PL (p=0.043) directions only
Conclusion
External proprioceptive feedback from the reach indicator improved DPC during the YBT and MYBT when compared to the MSEBT Sagittal plane COP velocities were reduced when external proprioceptive feedback from the reach indicator was present, while frontal plane COP velocities were not affected in this group of participants.
Corresponding Author
Kristen L Jagger, PT, MSPT, PhD
Professor | School of Physical Therapy Regis University 3333 Regis Blvd., G-4, Denver, CO 80221 P 303.964.6032 | F 303.964.5474 | PCH 403N | E kjagger@regis.edu | School of PT Website a
Dynamic postural control (DPC) describes an individual’s ability to maintain their balance within their base of support in both anticipatory and reactive balance situations.1 It can identify deficits, at-risk individuals, and inform prevention strategies. Position, velocity, and acceleration of the center of mass (COM) or center of pressure (COP) can be used as objective laboratory assessments of DPC. Yu and colleagues2 highlighted COM acceleration as a convenient measure of postural control, while Masani and colleagues3 demonstrated that COP velocity most accurately reflects the acceleration of COM. These studies collectively support the use of COP velocity to describe balance abilities during DPC assessments. COP velocity has successfully differentiated between static balance abilities of male non-athletes and similarly aged male soccer athletes who had lower COP velocities, suggesting greater balance control.4
Common standardized DPC assessments for healthy active adults include the modified Star Excursion Balance Test (MSEBT) and the Y-Balance Test of the Lower Quarter (YBT). Both tests have been used to measure dynamic balance in athletes and healthy active adults, but the outcomes of the tests are not equivalent.5‑8 A modification of the YBT, the Modified Y-Balance Test (MYBT), has also been evaluated to determine if centralizing the location of input on the YBT reach indicator would create more consistent outcomes between the MSEBT and YBT 9 Findings from that study revealed similar reach distance outcomes between the YBT and MYBT, but not between the YBT/MYBT and the MSEBT It was proposed this discrepancy may have been due to the MSEBT’s use of a feedforward motor control strategy due to the lack of a reach indicator, while the YBT and MYBT used a feedback strategy due to the sensory input received from the reach indicator during testing.6,9
It is clear that reach distances vary between the YBT/ MYBT and MSEBT, yet there is still a need for further clarity regarding the reason for these differences. If there is merit to the supposition that continuous feedback from the reach indicator is responsible for the increase in reach distances during performance of the YBT/MYBT, it follows that the reach indicator enhances DPC, which may or may not be desired by the examiner. If DPC is enhanced, one would expect to see slower COP velocities during performance of the YBT/MYBT when compared to the MSEBT Therefore, the purpose of this study was to explore DPC during performance of the MSEBT, the YBT, and a modified version of the YBT, the MYBT. The directional hypothesis stated that COP velocities recorded during the YBT and MYBT would be slower than those recorded during the MSEBT due to the presence of the reach indicator as a feedback mechanism.
This was a multivariate cross-sectional study that evaluated the differences between COP velocities in multiple planes between three tests (e.g., YBT, MYBT, and MSEBT) and in three reach directions (e.g., anterior [AN], posteromedial [PM], and posterolateral [PL]).
Approval was obtained from the university’s Institutional Review Board (IRB) prior to participant recruitment. A convenience sample of 21 participants was recruited from a pool of healthy, young individuals from the university population. Participants were included if they were healthy adults aged 18-35 years with no history of lower extremity injuries in the previous six months or diagnosed neurological or balance disorders. Participants were excluded from the study if any of the following were present: lower extremity amputation, history of lower extremity fracture, vestibular disorders, undergoing current treatment for inner ear/sinus/upper respiratory infection, concussion within the prior three months, past medical history of surgery for a lower extremity injury within the prior six months, currently pregnant or think they may be pregnant, or medically prohibited from participating in physical activities. Before engaging in data collection, participants read a description of the study, were offered an opportunity to ask questions, and signed a consent form. YBT, MSEBT, and MYBT reach performance data from participants in this study have been published previously,9 but COP data have not been included in any other published manuscript.
Each participant was oriented to the balance tests, bilateral lower extremity leg lengths were measured for normalizing reach outcomes, three practice trials of each assessment were performed, and a two-minute rest period was taken before formal testing. The order of the three balance tests was randomized to minimize the impact of fatigue and learning effect. Each test was scored by the same researcher who was certified to administer the YBT through Functional Movement Systems™ (Danville, VA). Prior researchers have demonstrated good to excellent intra-rater reliability (0.85-0.91)10 when the YBT was performed by trained examiners.
Participants completed all three balance tests during a single testing session. Performances were normalized using leg length, and three trials of each reach direction – AN, PM, and PL – were recorded on each lower extremity All testing was performed barefoot and with the YBT stance plate on a single force plate (AMTI, Inc., Watertown, MA. USA). COP velocities for frontal plane (medial-lateral) and sagittal plane (anterior-posterior) directions were sampled at 1200 Hz and filtered with a low pass Butterworth filter at 12 Hz.
Per the YBT protocol, participants were instructed to begin by standing on the right leg with the foot centered on the stance plate and toes behind a pre-set line, and to push the reach indicator in the red target area toward the direction being tested (Figure 1a). Participants were instructed to place their hands on their hips and maintain the heel of the stance leg in contact with the stance plate while per-
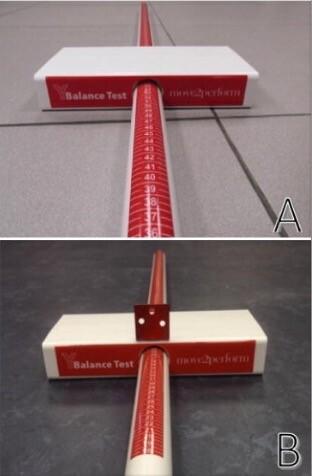
forming each reach. Reach distance was measured at the trailing edge of the reach indicator to the nearest centimeter. Trials were discarded and repeated if the participant’s reach foot touched the floor or kicked the reach indicator, if the stance heel was lifted from the stance plate, or the participant failed to return to the start position in a controlled manner.
In contrast to the YBT, during the MYBT, participants pushed the reach indicator by using an additional fabricated tab that was centered on the superior surface of the reach indicator and aligned with its trailing edge. The fabricated tab (Figure 1b) was attached to the top of the reach indicator such that the reach foot was centered over the reach indicator and was effectively reaching at the level of the stance foot and at the midline of each reach direction, which is spatially more similar to the MSEBT Trials were considered invalid for the same reasons listed for the YBT.
To perform the MSEBT, the participants stood on the YBT stance plate and followed the same protocol as the YBT but did not slide a reach indicator. Instead of pushing the reach indicator, participants reached out and lightly touched the YBT frame with the reach foot in each of the three testing directions. Performance of the MSEBT on the YBT frame was deemed necessary to minimize the effect of perceptual differences associated with standing on a raised surface versus the floor The distances were recorded using the same measuring system as the YBT. Trials were deemed invalid for the same reasons as listed for the YBT
1. Participant Demographics Females
(n=9) (n=11)
Prior to conducting this study, an a priori power analysis was conducted to determine the necessary sample size using G*Power 3.1 (© 2010-2019 Heinrich Heine Universität Düsseldorf). Calculations indicated that a sample size of 21 was necessary to achieve 80% power COP velocities were unsigned to appreciate magnitude from each axis as a positive number, regardless of direction. Average COP velocities were calculated for the frontal plane (COPx), the sagittal plane (COPy), and the resultant of these two planes (COPr). A 3-way analysis of variance (ANOVA) was used to determine differences between COP velocities across tests (YBT, MYBT, SEBT), reach directions (AN direction, PM direction, PL direction), and sides (left and right). Tukey’s HSD post hoc analyses were conducted to further identify differences. IBM SPSS Statistics 28.0.0.0 was used for all statistical analyses.
Twenty-one subjects participated (9 females, 11 males, mean age 24.5 ± 1.2 years) (Table 1).
Analysis of variance results revealed a significant main effect for both test (F=4.485, p<0.001) and reach direction (F=61.594, p<0.001) but no significant finding for side (F=2.075, p=0.102). Post hoc analyses for test indicated significant differences in COPy (sagittal plane) between YBT and MSEBT (p=0.034) and between MYBT and MSEBT (p<0.001), as well as significant differences in COPr between MYBT and MSEBT (p=0.002). Post hoc analyses for reach direction revealed significant differences in COPx (frontal plane) between AN and both PM (p<0.001) and PL (p<0.001) directions, in COPy between AN and PM (p<0.001) and PL (p<0.001) directions, and COPr between AN and PL (p=0.043) directions only (Table 2).
Data specific to each test and reach direction are graphically summarized by frontal plane COP velocities (Figure 2), sagittal plane COP velocities (Figure 3), and the resultant COP velocities (Figure 4).
FRONTAL PLANE (COPX)
There was no significant difference in frontal plane COP velocities between any of the three balance tests, but velocities were significantly slower across all tests during performance of the anterior reach (Figure 2). The lack of lower frontal plane COP velocities in the presence of an external feedback mechanism, regardless of foot contact location (YBT/MYBT), does not support the directional hypothesis
Table 2. Center of Pressure Velocities by Plane Across Tests and Reach Directions.
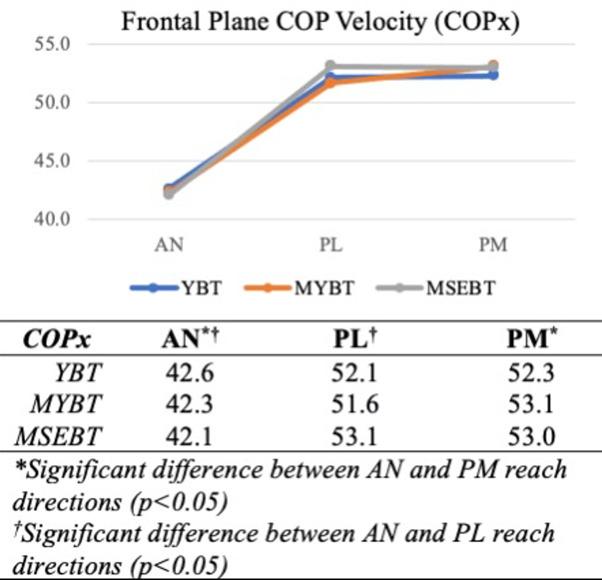
that a feedback loop would improve DPC. The significantly slower frontal plane COP velocities during the performance of the anterior reach is consistent with the direct sagittal plane reaching motion, in which primary sagittal plane COP velocities are expected. This is also the only direction in which the participants could consistently visualize the reach foot throughout the motion, and visual input could have contributed to the enhanced frontal plane DPC seen in all three tests. Proprioceptive feedback does not alter frontal plane DPC during performance of the anterior reach.
Reaching in the posterior directions, regardless of the presence or absence of an external feedback mechanism, resulted in higher frontal plane COP velocities. The diagonal nature of this motion, blending frontal and sagittal planes, necessarily requires more frontal plane motion, yet the lack of differences in frontal plane COP velocities between tests is interesting. Prior research within the healthy
active adult population has demonstrated PM and PL reach distance performance differences between the YBT/MYBT and the MSEBT, where participants reached farther in the presence of feedback (YBT/MYBT).9 When considering the frontal plane COP velocities recorded in this study, it appears that greater reach distance performance does not necessarily correlate with greater frontal plane DPC.
Sagittal plane COP velocities were significantly lower, regardless of reach direction, during performance of the YBT and MYBT than during performance of the MSEBT (Figure 3). These findings support the directional hypothesis and agree with the previously proposed effects of an external feedback mechanism.6,9 The presence of feedback from the reach indicator, whether centralized (MYBT) or lateral to
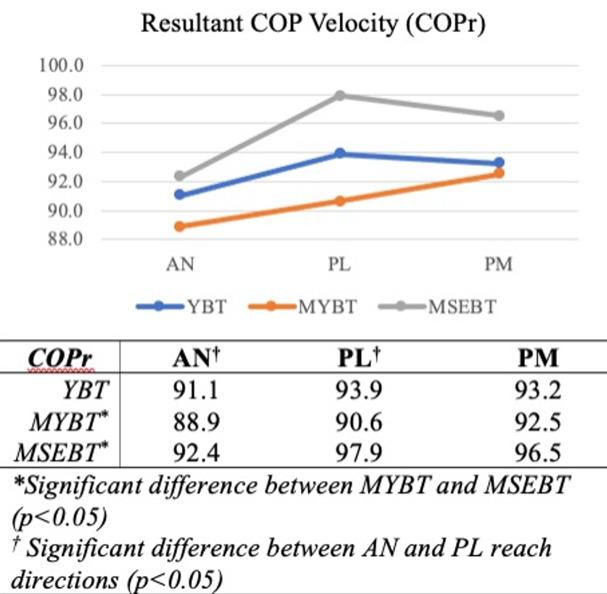
Figure 4. Resultant COP Velocities by Test and Direction
midline (YBT), improved sagittal plane DPC in this group of participants.
The participants in this study were healthy active adults who did not engage in regular sporting activities. Prior research has demonstrated differences in reach distance performance between the YBT/MYBT and the MSEBT within both healthy active adults9 and those participating regularly in sports.6,10 Within the healthy active adult population, both PM and PL reach performances were superior on the YBT/MYBT when compared to the MSEBT, while the anterior reach was not statistically different.9 Jagger and colleagues9 attributed this difference to the benefits of a feedback mechanism when vision of the target was limited. They further suggested that contradictory findings in an athletic population – in which YBT/MSEBT reach differences were only demonstrated in the AN direction6 – may have resulted from specific sports participation or training that enhanced proprioceptive awareness within posterior reaches where the target was not directly visible.9 Current data suggest feedback is more important for sagittal plane DPC, regardless of visual input or direction of reach, within healthy active adults.
Due to its representation of both frontal and sagittal planes, the resultant COP velocities demonstrated mixed findings (Figure 4). The resultant velocities recorded during performance of the MYBT were significantly different from the MSEBT, and AN versus PL velocities were significantly different. The loss of distinct patterns noted previously within frontal and sagittal planes is due to the creation of a resultant value that blends the two planar directions. The resultant velocities were specifically calculated to better represent the pattern of motion seen during the PM and PL reach directions – an oblique, or resultant, direction –
and better identify differences in those movements. While significant differences between the MYBT and MSEBT were not specific to the PL reach direction, a trend toward greater COPr velocity during performance of the MSEBT can be seen in Figure 4 During this motion, the reach foot and target are well out of the peripheral vision when participants reach their maximum, which would indicate that sagittal plane DPC and vision are more critical to performance of this task. Ultimately, the COPr velocity findings presented here blur the differences demonstrated by a more planar approach, even in movements that are more oblique in nature.
In summary, greater DPC was exhibited during performance of the YBT/MYBT when compared to the MSEBT, which agreed with the directional hypothesis. Slower sagittal plane COP velocities were recorded when the reach indicator was present and supports the suggested proprioceptive feedback mechanism. Having a constant proprioceptive feedback loop during the outward reaching motions allowed for greater DPC and resulted in the previously reported higher reach distances on the YBT/MYBT 9
This study has several limitations. The sample size is small, which limits generalizability of the findings. Standing on the YBT stance plate did provide for a consistent position from which to record measurements for each test and reach direction, but it did not account for the non-standard elevated surface used for the MSEBT, which may have altered visual perceptions and testing outcomes.
A comprehensive assessment of COP velocity data from the YBT, MYBT, and MSEBT in this population of healthy active adults reveals the importance of external proprioceptive feedback on sagittal plane DPC. The presence of external proprioceptive feedback from the reach indicator had a greater effect on sagittal plane DPC than frontal plane DPC. Vision may have contributed to DPC when the reach foot was visible. Selection of a DPC assessment tool should be based upon the population of interest and the types of functional activities they engage in. Based on current results from healthy active adults, use of the MSEBT would provide a greater challenge to sagittal plane DPC due to its lack of a feedback mechanism.
The authors report no conflicts of interest.
The authors would like to thank Anna Critz, Cara Delp Grubb, Amanda Frazier, and Maggie Phillips Vencille for their assistance with participant recruitment and data collection during their graduate studies.
Submitted: December 15, 2023 CDT, Accepted: May 29, 2024 CDT
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Welch TDJ, Ting LH. Mechanisms of motor adaptation in reactive balance control. PLoS ONE 2014;9(5):e96440. doi:10.1371/journal.pone.0096440
2. Yu E, Abe M, Masani K, et al. Evaluation of postural control in quiet standing using center of mass acceleration: comparison among the young, the elderly, and people with stroke. Arch Phys Med Rehab 2008;89(6):1133. doi:10.1016/j.apmr.2007.10.047
3. Masani K, Vette AH, Abe MO, Nakazawa K. Center of pressure velocity reflects body acceleration rather than body velocity during quiet standing. Gait Posture. 2014;39(3):946-952. doi:10.1016/ j.gaitpost.2013.12.008
4. Thompson LA, Badache M, Cale S, Behera L, Zhang N. Balance performance as observed by center-ofpressure parameter characteristics in male soccer athletes and non-athletes. Sports (Basel) 2017;5(4). doi:10.3390/sports5040086
5. Bulow A, Anderson JE, Leiter JR, MacDonald PB, Peeler J. The modified star excursion balance and ybalance test results differ when assessing physically active healthy adolescent females. Int J Sports Phys Ther 2019;14(2):192-203.
6. Coughlan GF, Fullam K, Delahunt E, Gissane C, Caulfield BM, Sci M. A comparison between performance on selected directions of the star excursion balance test and the Y Balance test. J Athl Train 2012;47(4):366-371.
7. Gabriel EH, Powden CJ, Hoch MC. Comparison of the Y-Balance test and star excursion balance test: utilization of a discrete event simulation. J Sport Rehabil. 2020;30(2):214-219. doi:10.1123/ jsr.2019-0425
8. Powden CJ, Dodds TK, Gabriel EH. The reliability of the star excursion balance test and lower quarter ybalance test in healthy adults: a systematic review Int J Sports Phys Ther 2019;14(5):683-694.
9. Jagger K, Frazier A, Aron A, Harper B. Scoring performance variations between the y-balance test, a modified y-balance test, and the modified star excursion balance test. Int J Sports Phys Ther. 2020;15(1):34-41.
10. Plisky PJ, Gorman PP, Butler RJ, Kiesel KB, Underwood FB, Elkins B. The reliability of an instrumented device for measuring components of the star excursion balance test. N Am J Sports Phys Ther. 2009;4(2):92-99.

Mehta N, Acuna AJ, McCormick JR, et al. Publicly Available Anatomic Total Shoulder Arthroplasty Rehabilitation Protocols Show High Variability and Frequent Divergence from the 2020 ASSET Recommendations. IJSPT. Published online July 1, 2024:856-867. doi:10.26603/001c.118926
Nabil Mehta1 , Alexander J Acuna1 , Johnathon R McCormick1 , William E Harkin1a , Hasani W Swindell2 , Steven F Defroda3 , Mike Reinold4 , Gregory P Nicholson1 , Grant E Garrigues1
1 Department of Orthopaedic Surgery, Rush University, 2 Department of Orthopaedic Surgery, Columbia University, 3 Department of Orthopaedic Surgery, University of Missouri, 4 Independent Researcher
Keywords: total shoulder arthroplasty (TSA), rehabilitation protocol, American Society of Shoulder and Elbow Therapists (ASSET), American Shoulder and Elbow Surgeons (ASES), range of motion (ROM), ASSET consensus statement, total, shoulder, arthroplasty https://doi.org/10.26603/001c.118926
Background
In 2020, the American Society of Shoulder and Elbow Therapists (ASSET) published an evidence-based consensus statement outlining postoperative rehabilitation guidelines following anatomic total shoulder arthroplasty (TSA).
The purpose of this study was to (1) quantify the variability in online anatomic TSA rehabilitation protocols, and (2) assess their congruence with the ASSET consensus guidelines.
This study was a cross-sectional investigation of publicly available, online rehabilitation protocols for anatomic TSA. A web-based search was conducted in April 2022 of publicly available rehabilitation protocols for TSA. Each collected protocol was independently reviewed by two authors to identify recommendations regarding immobilization, initiation, and progression of passive (PROM) and active range of motion (AROM), as well as the initiation and progression of strengthening and post-operative exercises and activities. The time to initiation of various components of rehabilitation was recorded as the time at which the activity or motion threshold was permitted by the protocol. Comparisons between ASSET start dates and mean start dates from included protocols were performed.
Results
Of the 191 academic institutions included, 46 (24.08%) had publicly available protocols online, and a total of 91 unique protocols were included in the final analysis. There were large variations seen among included protocols for the duration and type of immobilization post-operatively, as well as for the initiation of early stretching, PROM, AROM, resistance exercises, and return to sport. Of the 37 recommendations reported by both the ASSET and included protocols, 31 (83.78%) were found to be significantly different between groups (p<0.05).
Corresponding Author:
William Harkin, MD
Department
of Orthopaedic Surgery
Rush University Medical Center 1611 W Harrison St, Suite 360 Chicago, IL 60621
Phone: 916-751-6517
Email: willharkin22@gmail.com
Considerable variability was found among online post-operative protocols for TSA with substantial deviation from the ASSET guidelines. These findings highlight the lack of standardization in rehabilitation protocols following anatomic TSA.
The utilization of anatomic total shoulder arthroplasty (TSA) in the United States has increased dramatically in recent years, with growth projected to outpace that of total hip and knee arthroplasty by 2025.1‑3 As the rate of TSA procedures continues to rise, there has been considerable modification to surgical technique, implant design, and peri-operative care strategies.4 Specifically, there has been ongoing debate regarding the optimal rehabilitation protocol after TSA.1
Adherence to a postoperative rehabilitation protocol has long been regarded as essential in optimizing patient outcomes after TSA.5,6 Generally, rehabilitation is broken into three phases: passive range of motion and stretching, active range of motion with isometric exercises, and resistance exercises with progression of higher-level activities. Despite this, the current literature reports significant heterogeneity in rehabilitation activities and timelines.7 A recent review of 16 studies found significant variability between rehabilitation protocols after TSA, with strategies based on biomechanical principles rather than clinical milestones.1 Furthermore, 75% of the included studies were Level 5 evidence, highlighting the paucity of high-quality evidence available to guide rehabilitation practices. Limitations in the available literature have led providers and institutions to create their own rehabilitation protocols and guidelines, many of which are published online in order to increase clarity and coordination of care for patients and their therapists. However, the variability and abundance of these protocols may lead to additional confusion among patients and therapists.
The American Society of Shoulder and Elbow Therapists (ASSET) published an evidence-based consensus statement in 2020, outlining postoperative rehabilitation guidelines following anatomic TSA.8 These recommendations are based on a rigorous synthesis of practice patterns from ASSET members and American Shoulder and Elbow Surgeons (ASES) members. Despite the availability of this consensus statement, it is unclear to what extent existing rehabilitation protocols align with these recommendations.
The purpose of this study was to (1) quantify the variability in online anatomic TSA rehabilitation protocols, and (2) assess their congruence with the ASSET consensus guidelines. The authors hypothesized that there would be significant variability among published protocols, and that there would be significant divergence between these protocols and the 2020 ASSET consensus statement.
This study was a cross-sectional investigation of publicly available, online rehabilitation protocols following anatomic TSA. A web-based search was conducted on April 1st , 2022, of publicly available rehabilitation protocols for TSA from websites of all Accreditation Council for Graduate Medical Education (ACGME)–accredited academic orthopaedic institutions identified on the Electronic Residency Application Service (ERAS). Protocols were identified on the institutions’ websites. If they could not be located, a Google search using the search term “[Program/hospital affiliate/medical school affiliate] total shoulder arthroplasty rehabilitation protocol” was performed.
To supplement the original search, a second Google query was performed using the general terms “total shoulder arthroplasty rehabilitation protocol”, “total shoulder replacement rehabilitation protocol”, and “TSA rehabilitation protocol” to identify programs published online from private practices, individual practitioners, or non-academic institutions. TSA rehabilitation protocols appearing on the first 10 pages (corresponding to the first 100 hits on Google) of this query’s results were included. Articles were included from this secondary search if they commented on rehabilitation protocols following anatomy TSA. Duplicate protocols, those not in English, or those published outside the United States were excluded. Figure 1 demonstrates a flow diagram demonstrating protocol selection.
Two separate reviewers independently conducted all searches and reviewed related protocols for inclusion. Disagreements among these reviewers were settled by a third independent reviewer. Each collected protocol was similarly independently reviewed by two authors to identify recommendations regarding immobilization, initiation, and progression of passive and active range of motion (ROM), initiation and progression of strengthening, and post-operative exercises and activities. The time of initiation was recorded as the time at which the activity or motion threshold was permitted by the protocol. Therefore, all time-related metrics reported as ranges were recorded as the early limit (i.e., 4-6 weeks was recorded as 4 weeks). Activities permitted within the first post-operative week were recorded as week 1.
Metrics for range of motion included time to initiation of passive ROM and active ROM, as well as the time after which various motion thresholds were permitted. Various components of ROM were collected as specified by each
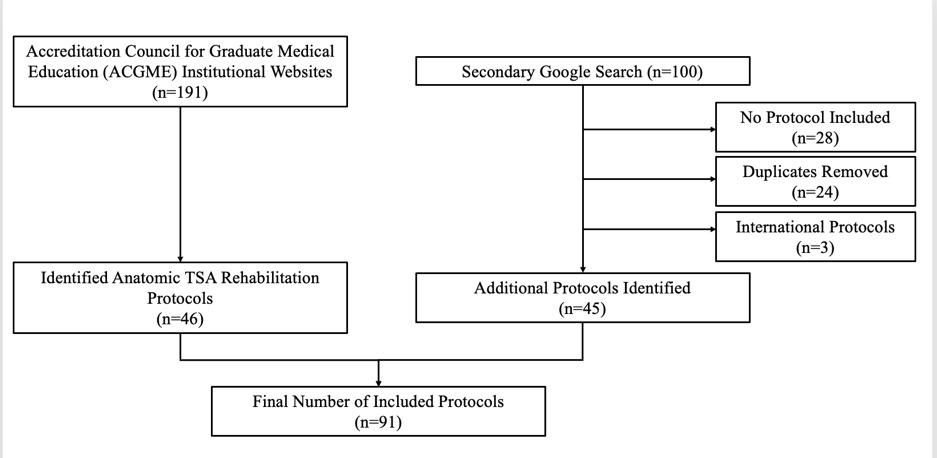
Figure 1. Flowchart demonstrating the breakdown of how protocols were identified and included/excluded for the study
protocol, including forward flexion, abduction, external rotation, and internal rotation.
Strength metrics were recorded when explicitly permitted by each protocol. Collected metrics included time to initiation of strengthening, strengthening modalities (including isometric versus resistance exercises), specific exercises permitted by the protocol, and return to higher level activities such as sports.
All statistical analyses were conducted in Microsoft Excel (version 16.51; Microsoft Corp., Redmond, WA, USA). Descriptive statistics including means, ranges, and standard deviations (SD) of the initiation times for passive and active ROM thresholds, immobilization, and exercises were performed. Percent recommending various active and resistance exercises and sports specific activities were summarized as percentages of all reporting protocols. Comparisons between ASSET start dates and mean start dates from included protocols were performed. One sample t-tests were conducted to compare mean (SD) start dates and ASSET recommendations with resulting p-values reported. Statistical significance was set at p<0.05.
Of the191 academic institutions queried, 46 (24.08%) had publicly available protocols online. The remaining 45 protocols were identified following the secondary search query which resulted in a total of 91 protocols in included in the analysis.
Table 1. Postoperative Adjunct Therapy Recommendations.
A total of 81 (89.01%) of included protocols commented on immobilization. Table 1 displays a breakdown of mode of immobilization, with most protocols utilizing a sling alone (n=53, 58.24%) or a sling with an immobilizer and/or abduction pillow (n=26; 28.57%). A total of 10 (10.99%) of protocols specified the use of a sling and abduction pillow in accordance with the ASSET recommendations. Of the 72 (79.12%) protocols addressing sling discontinuation, the average (± SD) reported time to discontinuation was 5.31 ± 1.18 weeks (ASSET recommendation: five weeks; p=0.03). Average time of complete immobilization was two weeks, however only one center recommended a period of complete immobilization.
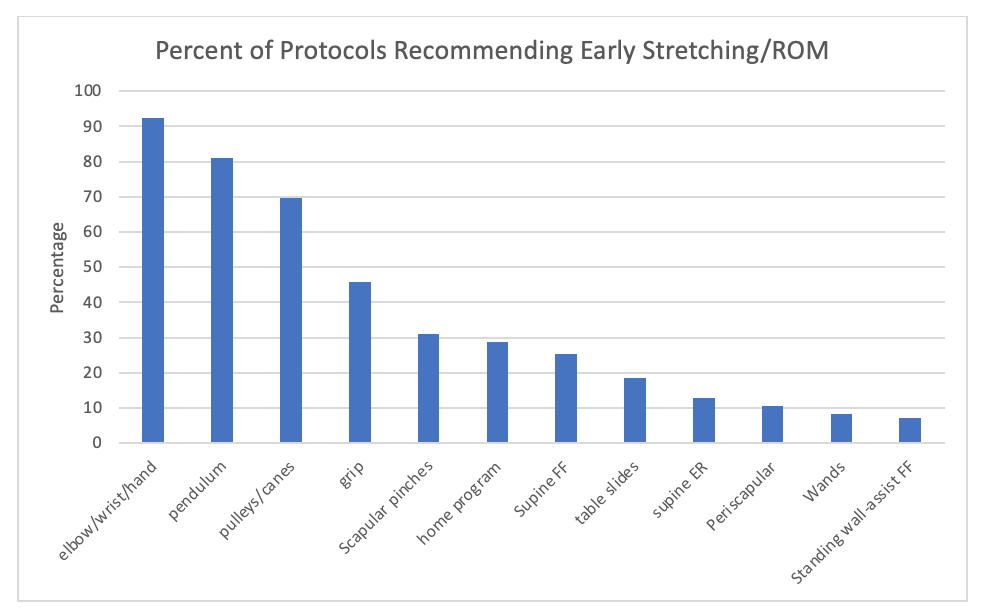
Figure 2. Percent of programs recommending stretching or range of motion.
Exercises that were recommended by fewer than 5% of programs were excluded (standing upper trap stretch, standing IR behind back, cross-body stretch, IR behind back stretch, supine abduction and sleeper stretch). ROM = Range of Motion. FF=Forward flexion. ER=External Rotation. IR=Internal Rotation.
The most commonly reported early ROM exercises can be found depicted in Figure 2. “Early” was defined by initiation before six weeks. The majority of programs recommended early elbow/wrist/hand ROM (n=81, 89.01%) and pendulum exercises (n=71, 78.02%), as well as pulley/cane exercises (n=61, 67.03%). However, there was wide variability with respect to the recommended start dates for these exercises (Figure 3). The mean (±SD) start date was significantly later among included protocols for the initiation of pulleys/canes (3.05 ± 1.87 vs. one week; p<0.01) and table slides (2.31 ± 1.85 vs. one week; p=0.01) compared to ASSET recommendations.
There was wide variability regarding when programs recommended initiating various passive ROM (PROM) exercises (Figure 4). Included protocols recommended starting any passive ROM at an average of 1.19 weeks postoperatively (range: 1-6 weeks). Passive forward flexion (PFF) was initiated at an average of 1.20 weeks (range: 1-6 weeks) with passive external rotation initiated at an average of 1.21 weeks (range: 1-6 weeks). Passive abduction (PAb) was initiated at 1.35 weeks (range: 1-6 weeks) and passive internal rotation (PIR) was begun at an average of 1.65 weeks (range: 1-6 weeks). Unrestricted passive ROM was permitted at an average of 7.08 weeks postoperatively (range: 1-13 weeks) (Figure 4).
There were significant differences between the mean (±SD) start date among included protocols and ASSET rec-
ommendations for a majority of start dates for various PFF, PER, PIR, and PAb cut-offs (Figure 4). Notably, unrestricted PROM was significantly later for the ASSET recommendation (7.08 ± 2.64 vs. 12 weeks; p<0.01).
Similarly, there was wide variability among start dates for active ROM (Figure 5). Active ROM was initiated at an average of 3.96 weeks (range: 1-9 weeks), active elevation/forward flexion at 3.94 weeks (range: 1-9 weeks) and active abduction at 3.87 weeks (range: 1-6 weeks). Active external rotation was initiated at an average of 4.07 weeks (range: 1-9 weeks), active internal rotation at 5.40 weeks (range: 1-12 weeks) and unrestricted active ROM allowed at an average of 10.29 weeks (range: 4-16 weeks) postoperatively.
Reported values for active ROM were significantly different than the ASSET recommendation for the initiation of active ROM (3.96 ± 1.95 vs. 7 weeks; p<0.001), active IR behind the back (7.90 ± 2.02 vs. 5 weeks; p=0.001), and unrestricted active ROM (10.29 ± 2.68 vs. 12 weeks; p<0.001).
The most commonly recommended resistance exercises included isometric external rotation, isometric abduction, resisted forward flexion, resisted external rotation and resisted internal rotation (Figure 6). There was wide variability in the way these exercises were be performed (i.e., bands, dumbells). There was also considerable variability in the reported start dates for these exercises (Figure 7). The start dates for the initiation of resistance exercises recommended by the ASSET consensus protocol were sig-
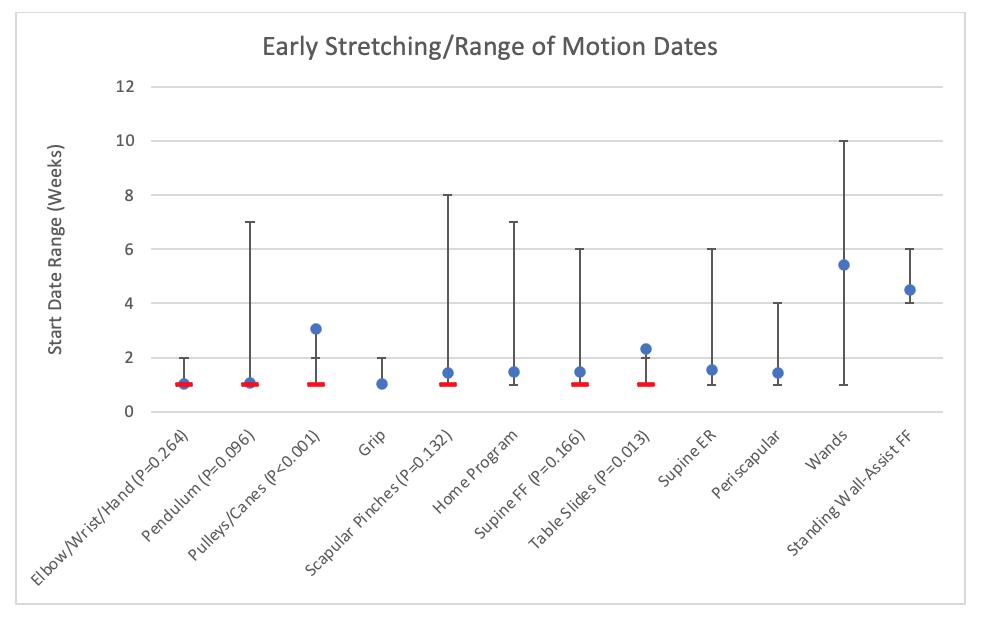
Figure 3. Range of start dates for early stretching or range of motion (ROM).
Exercises that were recommended by fewer than 5% of programs were excluded (standing upper trap stretch, standing IR behind back, cross-body stretch, IR behind back stretch, supine abduction and sleeper stretch). P-values represent comparisons between the mean (SD) start date ranges among included protocols (blue dot) and ASSET recommendations (red dash). FF=Forward flexion. ER=External Rotation. IR=Internal Rotation.
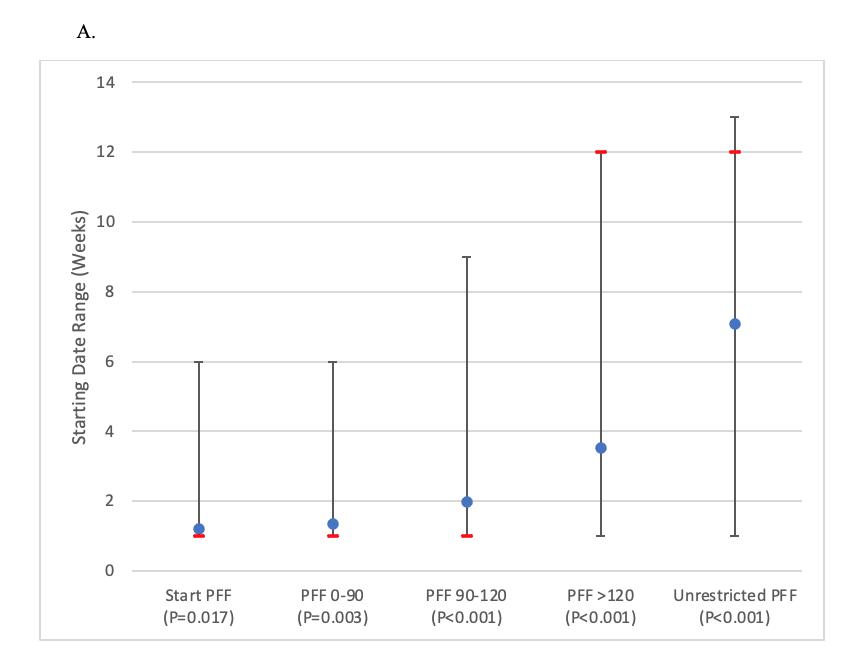
Figure 4 (A-D). Passive A. Forward Flexion (PFF), B. Abduction (PAb), C. External Rotation (PER), and Internal Rotation (PIR) range of motion start dates (with range of minimum and maximum values reported).
P-values represent comparisons between the mean (SD) start date ranges among included protocols (blue dot) and ASSET recommendations (red dash).
nificantly later than the mean (±SD) reported among included protocols. Specifically, there were differences seen for the start date of band training (6.97 ± 2.42 vs. 12 weeks; P<0.01), bicep curls (6.50 ± 2.38 vs. 12 weeks; P<0.01), up-
per extremity closed chain exercises (9.58 ± 3.48 vs. 12 weeks; P<0.01), and dumbbells for shoulder strengthening (8.79 ± 3.83 vs. 12 weeks; P<0.01).

Figure 5. Start dates for active range of motion.
P-values represent comparisons between the mean (SD) start date ranges among included protocols (blue dot) and ASSET recommendations (red dash). ROM Range of Motion. FF=Forward flexion. ER=External Rotation. IR=Internal Rotation.
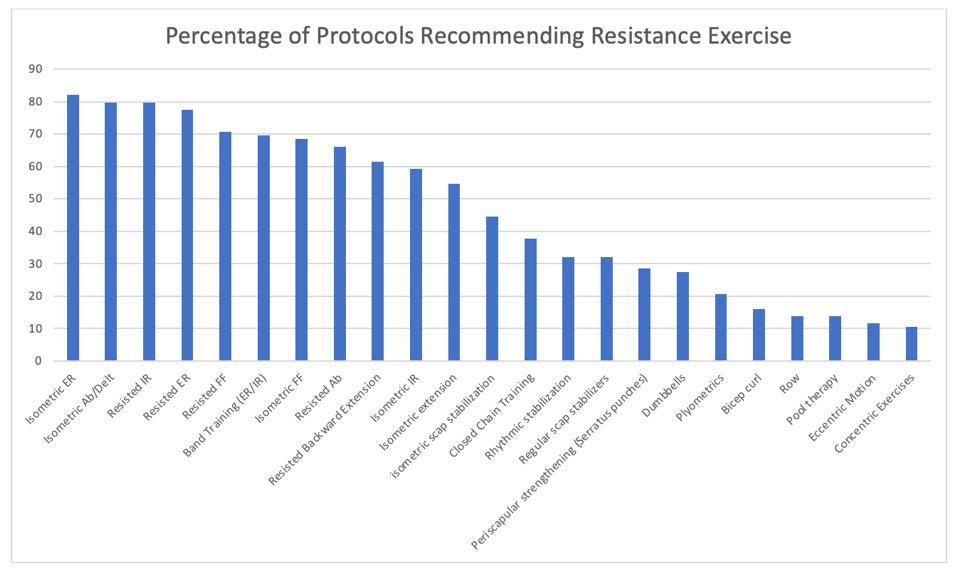
Figure 6. Percentage of programs recommending resistance exercise. Exercises with less than 10% of programs recommending were excluded (dynamic hug, wall slides, ball stabilization, wall pushups, Upper extremity (UE) bike, ball toss, standing forward punch).
RETURN TO WORK/SPORTS AND ACTIVITIES OF DAILY LIVING
The included protocols demonstrated significant variation in the time to return to work (average 15.50, range: 6-24 weeks). Some sport specific activities were recommended (Figure 8). Return to golf and other sport specific activities without overhead components averaged 15.53 weeks
(range: 10-24 weeks), with contact sports initiated at an average of 24.00 weeks postoperatively (Figure 9). Return to previous activity level occurred on average at 19.50 weeks (range: 12-23 weeks) and return to independent activities of daily living at 10.36 weeks (range 6-16 weeks). Protocols reported initiation dates for golf/sport specific activities (non-overhead) (15.53 ± 3.42 vs. 12 weeks; p<0.01), over-
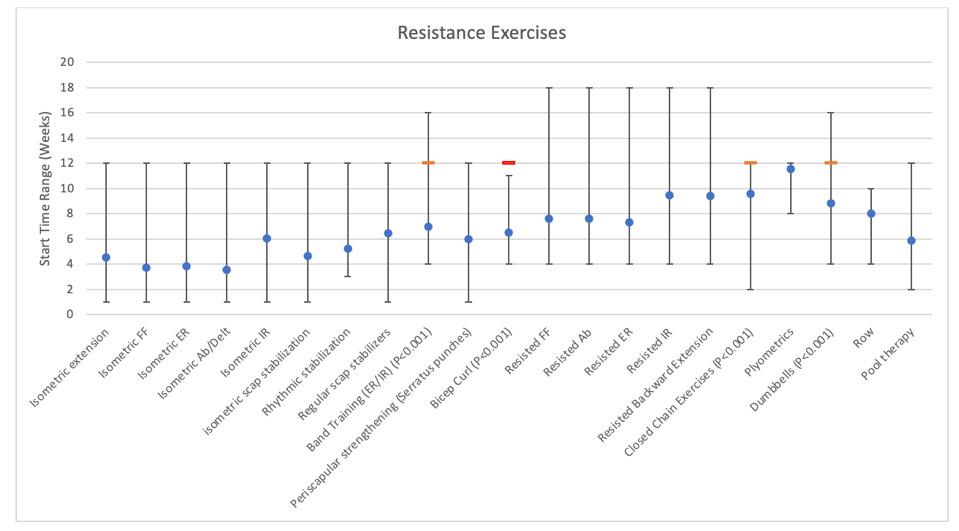
Figure 7. Average recommended start dates with range (minimum and maximum) for resistance exercises. Exercises with less than 10% of programs recommending were excluded (dynamic hug, wall slides, ball stabilization, wall pushups, UE bike, ball toss, standing forward punch). P-values represent comparisons between the mean (SD) start date ranges among included protocols (blue dot) and ASSET recommendations (red dash). FF=Forward flexion. ER=External Rotation. IR=Internal Rotation. Ab=Abduction.
head sports activities (including tennis) (16.80 ± 4.02 vs. 12 weeks; p<0.01), swimming (16.67 ± 5.10 vs. 12 weeks; p=0.03), return to previous level of activity (19.5 ± 5.13 vs. 24 weeks; p=0.04), and independent activities of daily living (10.36 ± 3.11 vs. 5 weeks; p<0.01) that were significantly different than those recommended by the ASSET
This investigation was focused on assessing the variability across online postoperative anatomic total shoulder arthroplasty rehabilitation protocols as well as their concordance with published ASSET guidelines. The authors hypothesized that there would be significant variability among published protocols, and that there would be significant divergence between these protocols and the 2020 ASSET consensus statement. Overall, considerable variability was found amongst current online protocols in terms of the time course for the initiation of specific planes of motion and strengthening exercises. Furthermore, substantial differences were found between these protocols and the ASSET guidelines, with surgeon protocols generally initiating activities at earlier timepoints than those recommended by ASSET
Protection of the subscapularis tendon is paramount during the early phase of healing after anatomic TSA. In accordance with the ASSET guidelines, a large majority of online protocols included the use of a sling for immobilization in
the post-operative period to facilitate this healing process. Interestingly, the average time for discontinuing the sling was longer among the included protocols than the ASSET guidelines, although this difference was small (5.31 weeks vs. 5 weeks) and perhaps not clinically significant. It is important to note that the consensus guideline supports discontinuation of a sling anywhere between the 4-to-6-week post-operative period given variability in subscapularis take-down methods and both pre- and intra-operative ROM assessments. This variability may help to explain why method and duration of immobilization differed from protocol to protocol, which has also been shown in prior studies.7
The timetable for the initiation of early ROM remains a subject of ongoing debate. While the average time to initiation of various ROM exercises was relatively comparable to that recommended by the ASSET protocol, there were many protocols that did not allow these movements until a later post-operative time. For example, although the initiation of supine forward flexion was recommended to start at post-operative week 1 by the ASSET protocol, multiple protocols did not allow for this movement until six weeks post-operatively Theoretically, prolonged immobilization has the potential to lead to stiffness. However, several authors have found no differences in patient reported outcomes or ROM in patients undergoing immediate or delayed therapy following shoulder arthroplasty at 12 months postoperatively 9,10 Although early mobilization is safe, it is not necessary to achieving a positive outcome in the long

Figure 8. Percentage of programs recommending postoperative sports specific activities.
ADLs=Activities of Daily Living
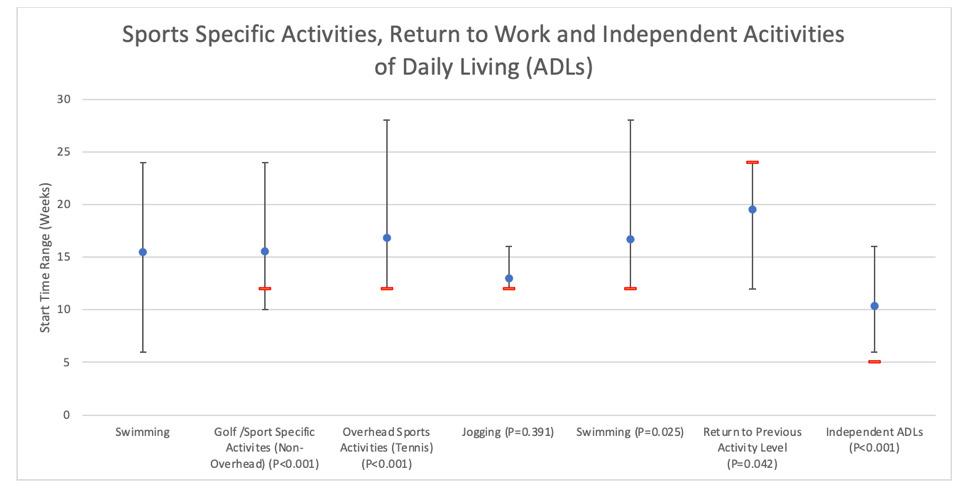
Figure 9. Recommended average start dates with range (minimum and maximum) for various postoperative sports specific activities.
P-values represent comparisons between the mean (SD) start date ranges among included protocols (blue dot) and ASSET recommendations (red dash). ADLs=Activities of Daily Living
term, but may have an overall impact on earlier improved function and quality of life within the first 12 months.
INITIATION OF PASSIVE AND ACTIVE RANGE OF MOTION
Based on the evidence-based protocol proposed by Jackins11 the ASSET recommendations for post-operative mobility progress from initial gentle stretching in Phase 1
to active assisted movement and fully active ROM during Phase 2 of rehabilitation (weeks 7 through 12). Similar to the trends regarding post-operative immobilization/early ROM, there were substantial inconsistencies between included protocols in the initiation of various passive and active ROM thresholds. For example, wide variability was seen in the start dates reported for unrestricted passive (range: 1 week to 13 weeks) and active (range: 4 weeks to 16 weeks) ROM. Similarly, the average values for this start date among included protocols were significantly earlier than the ASSET recommendations (unrestricted passive and active: 12 weeks). Furthermore, there were discrepancies among protocols regarding the angles reported for passive and active ROM goals. Notably, although the ASSET guidelines comment on passive external rotation to 30-40 degrees and to 60 degrees, included protocols reported nine different external rotation cutoffs (0-10, 15, 10-20, 25, 20-30, 30-40, 40-45, 45-60, 60). It is unclear if the nine different cutoffs were clinically significant versus the concept of assure a slow and gradual progression.
Early strengthening after anatomic TSA focuses on the periscapular musculature with gradual inclusion of rotator cuff exercises. As was demonstrated by Baumgarten et al., improvements in post-operative strength have been associated with greater improvements in ROM and patient reported outcomes.12 The inclusion of resistance training begins within Phase 3 of the ASSET rehabilitation consensus protocol, with strengthening primarily limited to below shoulder and frontal plane exercises. As was seen previously, wide variations were demonstrated for when each resistance exercise should be initiated, with a majority of included protocols reporting start dates within the first 2-3 months post-operatively As Phase 3 does not begin until post-operative week 12, significantly earlier start dates were seen for band training, bicep curls, closed chain exercises, and dumbbells among these protocols. However, these variations may be partly attributed to the limited information among the current literature regarding the exact timing and type of strengthening post-operatively.13 Furthermore, exercises against resistance can be initiated for many different reasons, including for neuromuscular activation and/or motor control. When designing protocols for these patients, specific attention should be paid to goals of each specific resistance exercise.
While rates of return to sport have generally been high following anatomic TSA,14‑18 there remains considerable variability in the current literature regarding the optimal time for this rehabilitation stage.13 Obviously, the details of the particular vocational or avocational activities influence timing of return. Although the ASSET recommends waiting until post-operative week 12 to initiate return to sports,19 full return is not indicated until post-operative month six to ensure adequate subscapularis healing. In a similar way,
ASSET allows gradual return to work at 12 weeks. However, given that strengthening begins in earnest at 12 weeks, many careers would dictate a more conservative timeline for actual return to work. Overall, for a majority of post-operative sports specific activities, the ASSET guidelines were significantly more conservative than start dates among the included protocols. Notably, although the ASSET consensus allows for swimming, non-overhead, and overhead sports to begin at post-operative week 12, the average initiation date for these activities for most protocols was after post-operative week 15. Conversely, included protocols allowed return to previous level of activity significantly earlier (i.e. at 12 weeks post-operatively), despite concerns related to glenoid loosening and failure associated with higher activity levels within this post-operative period.20
The investigation has several limitations to note. Despite the authors’ approach to identifying online protocols for analysis, there was a relative paucity of online protocols and as such, assuming that institutions have protocols that are not on the world wide web, the analyzed sample may not be representative of all ACGME institutions. Additionally, given the fairly recent publication of ASSET in 2020, there is a chance that current online protocols may be outdated and thus may not reflect the recent consensus guidelines. It is also possible that these websites have not been recently updated to reflect the current protocols of these institutions. Similar to a previously published comparison comparing online rehabilitation protocols following rotator cuff repair to ASSET guidelines, the nature of such an evaluation has limitations that warrant mention.21,22 The content of the ASSET statement was primarily developed by therapists with additional input from ASES surgeons.8 Although this procedure was inherent to the methodology for the development of these guidelines, there is the possibility for differing perspectives, opinions and approaches for surgeons and postoperative rehabilitation. This is also potentially true in the context of a large variety of prosthetic options presently available that may offer improved fixation strategies, mechanisms for bony in-growth of the implant, and options for subscapularis repair In addition to wide ranging surgical variables, there is also a wide range of patient variables in the TSA cohort. Each patient has unique combination of characteristics (age, activity level, comorbidities) which dictate the pace of their rehabilitation. Although the ASSET guidelines provide a consensus statement driven by current literature, they cannot be interpreted as the gold standard for postoperative rehabilitation for every patient. Perhaps this is one of the factors that led to such a wide variation between protocols analyzed in this study Regardless, the optimal protocol should have options for individualization based on each patient and their specific surgeon. To better elucidate specific time points for the initiation of ROM exercises and strengthening, high-quality literature is needed to assess muscle activation patterns, muscle force patterns and subsequent outcomes that might dictate when certain rehabilitation elements can be safely started.
Considerable variability was found among the included online post-operative protocols for anatomic TSA in terms of the time course for initiation of specific planes of motion and strengthening exercises. Notably, substantial deviation was found between these protocols and the ASSET guidelines. These findings highlight the lack of standardization in rehabilitation protocol following anatomic TSA. Subsequently, the current findings highlight the importance of a more standardized and specific rehabilitation protocol following anatomic TSA. Further investigation is warranted to assess the true impact of these variations and to identify optimal recommendations for the initiation of elements of postoperative rehabilitation.
There are no financial biases or conflicts of interest for any author related to this study. The study was exempt from International Review Board approval as no identifiable patient information was used.
Submitted: December 06, 2023 CDT, Accepted: May 04, 2024 CDT
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Kirsch JM, Namdari S. Rehabilitation after anatomic and reverse total shoulder arthroplasty: A critical analysis review. JBJS Rev. 2020;8(2):e0129. doi:10.2106/JBJS.RVW.19.00129
2. Wagner ER, Farley KX, Higgins I, Wilson JM, Daly CA, Gottschalk MB. The incidence of shoulder arthroplasty: rise and future projections compared with hip and knee arthroplasty J Shoulder Elbow Surg 2020;29(12):2601-2609. doi:10.1016/j.jse.2020.03.049
3. Palsis JA, Simpson KN, Matthews JH, Traven S, Eichinger JK, Friedman RJ. Current trends in the use of shoulder arthroplasty in the United States. Orthopedics 2018;41(3):e416-e423. doi:10.3928/ 01477447-20180409-05
4. Frank JK, Siegert P, Plachel F, Heuberer PR, Huber S, Schanda JE. The evolution of reverse total shoulder arthroplasty-From the first steps to novel implant designs and surgical techniques. J Clin Med. 2022;11(6). doi:10.3390/jcm11061512
5. Boardman ND 3rd, Cofield RH, Bengtson KA, Little R, Jones MC, Rowland CM. Rehabilitation after total shoulder arthroplasty J Arthroplasty 2001;16(4):483-486. doi:10.1054/arth.2001.23623
6. Mulieri PJ, Holcomb JO, Dunning P, et al. Is a formal physical therapy program necessary after total shoulder arthroplasty for osteoarthritis? J Shoulder Elbow Surg. 2010;19(4):570-579. doi:10.1016/ j.jse.2009.07.012
7 Bullock GS, Garrigues GE, Ledbetter L, Kennedy J. A systematic review of proposed rehabilitation guidelines following anatomic and reverse shoulder arthroplasty J Orthop Sports Phys Ther 2019;49(5):337-346. doi:10.2519/jospt.2019.8616
8. Kennedy JS, Garrigues GE, Pozzi F, et al. The American Society of Shoulder and Elbow Therapists’ consensus statement on rehabilitation for anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2020;29(10):2149-2162. doi:10.1016/j.jse.2020.05.019
9. Denard PJ, Lädermann A. Immediate versus delayed passive range of motion following total shoulder arthroplasty J Shoulder Elbow Surg 2016;25(12):1918-1924. doi:10.1016/j.jse.2016.07.032
10. Hagen MS, Allahabadi S, Zhang AL, Feeley BT, Grace T, Ma CB. A randomized single-blinded trial of early rehabilitation versus immobilization after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2020;29(3):442-450. doi:10.1016/ j.jse.2019.10.005
11. Jackins S. Postoperative shoulder rehabilitation. Phys Med Rehabil Clin N Am 2004;15(3):643-682. doi:10.1016/j.pmr.2004.01.002
12. Baumgarten KM, Osborn R, Schweinle WE Jr, Zens MJ. The influence of anatomic total shoulder arthroplasty using a subscapularis tenotomy on shoulder strength. J Shoulder Elbow Surg 2018;27(1):82-89. doi:10.1016/j.jse.2017.06.043
13. Liu JN, Steinhaus ME, Garcia GH, et al. Return to sport after shoulder arthroplasty: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2018;26(1):100-112. doi:10.1007/ s00167-017-4547-1
14. Bülhoff M, Sattler P, Bruckner T, Loew M, Zeifang F, Raiss P. Do patients return to sports and work after total shoulder replacement surgery? Am J Sports Med 2015;43(2):423-427 doi:10.1177/0363546514557940
15. Garcia GH, Liu JN, Mahony GT, et al. Hemiarthroplasty versus total shoulder arthroplasty for shoulder osteoarthritis: A matched comparison of return to sports. Am J Sports Med. 2016;44(6):1417-1422. doi:10.1177/ 0363546516632527
16. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery Am J Sports Med 2008;36(8):1577-1581. doi:10.1177/ 0363546508317126
17 Papaliodis D, Richardson N, Tartaglione J, Roberts T, Whipple R, Zanaros G. Impact of total shoulder arthroplasty on golfing activity. Clin J Sport Med. 2015;25(4):338-340. doi:10.1097/ JSM.0000000000000148
18. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty Am J Sports Med. 2010;38(10):2097-2105. doi:10.1177/ 0363546510371368
19. Brameier DT, Hirscht A, Kowalsky MS, Sethi PM. Rehabilitation strategies after shoulder arthroplasty in young and active patients. Clin Sports Med 2018;37(4):569-583. doi:10.1016/j.csm.2018.05.007
20. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280. doi:10.1016/j.jse.2010.06.007
21. Galetta MD, Keller RE, Sabbag OD, et al. Rehabilitation variability after rotator cuff repair. J Shoulder Elbow Surg 2021;30(6):e322-e333. doi:10.1016/j.jse.2020.11.016
22. Thigpen CA, Shaffer MA, Gaunt BW, Leggin BG, Williams GR, Wilcox RB 3rd. The American Society of Shoulder and Elbow Therapists’ consensus statement on rehabilitation following arthroscopic rotator cuff repair J Shoulder Elbow Surg 2016;25(4):521-535. doi:10.1016/j.jse.2015.12.018

Charles J Salvo
1a ,
Ashlie Crewe
1 ,
Dillon Estes
1 Physical Therapy, Lebanon Valley College
1
, Jessica Kroboth
1 ,
Celia Yost
1
Keywords: athletic performance, college athletes, pelvic floor dysfunction, pelvic floor muscle activity
https://doi.org/10.26603/001c.120211
International Journal of Sports Physical Therapy
Background
Pelvic floor dysfunction (PFD) occurs when muscles of the pelvic floor become weakened, impaired, or experience tension leading to a variety of complications. Due to the reactive nature and high demands of many sports, athletes are at increased susceptibility and of particular interest concerning PFD
Hypothesis/Purpose
The purpose of this study was to explore the prevalence of PFD among college-aged athletes, assess how PFD impacted athletic performance, and identify contributing factors for increased likelihood of PFD in athletes.
Study Design
Cross-Sectional Study
Methods
All fully active LVC NCAA Division 3 athletes were recruited for screening for PFD using the Cozean Pelvic Dysfunction Screening Protocol and were surveyed on their self-knowledge of PFD Athletes who scored ≥ 3 on this tool completed an additional survey, created by the investigators, to identify the impact PFD had on their athletic performance and personal life and were then randomly assigned to one of three investigators to undergo a noninvasive coccygeal assessment to determine underactive, overactive, or normal pelvic floor muscle (PFM) activity
Results
Fifty-three Division III male and female athletes between the ages of 18-25 years old participated in the study Statistically significant differences were found between Cozean scores and demographic factors of age (p <0.001), gender (p <0.05), self-knowledge of PFD (p <0.001), and sport (p <0.001) among all participants that contributed to the increased likelihood of PFD Thirteen athletes scored ≥ 3 on the Cozean with the 92.3% experiencing under/over active PFM activity and the majority indicating that PFD significantly impacted their athletic performance and quality of life.
Conclusion
The results indicate that older female NCAA Division III college athletes who participate in swimming and who possess self-knowledge of PFD are more likely to experience PFD Additionally, these athletes are likely to encounter a significant impact on their athletic performance and quality of life. These results provide preliminary evidence on the need of PFD awareness and assessment among college athletes.
Charles J Salvo, Department of Physical Therapy, Lebanon Valley College, Annville, PA 17003, United States of America. Telephone number: 570-510-0503. Fax number: 717-867-6673. Email address: salvo@lvc.edu
The pelvic floor and its associated musculature function to support and stabilize surrounding structures, aid in sexual function, act as a sphincter, assist in lymphatic return, and affect posture and breathing mechanics.1 The pelvic floor musculature (PFM) is deeply interconnected with the rest of the human anatomy and functions similarly to other skeletal muscles.2 As a result, injury or dysfunction can occur Pelvic floor dysfunction (PFD) occurs when the structures of the pelvic floor are weakened, tense, or impaired, leading to a variety of symptoms and complications.3 Common symptoms of PFD include, but are not limited to, urinary (most common) or anal incontinence, pelvic pain, and sexual dysfunction.4,5 To treat PFD, physical therapists use manual therapy, strengthening, and conditioning through exercise prescription to mitigate pain and other symptoms.6 Pelvic floor physical therapists treat a variety of other conditions including general PFM (classified as underactive or overactive), endometriosis, vaginismus, pelvic organ prolapse, and pre/post-natal care to name a few.7
Athletes are of particular interest when it comes to PFD Rebullido, et al. found that high-impact sports involving jumping, landing, or running show the highest prevalence rates of urinary loss among young female athletes.8 Additionally, Rodríguez-López, et al. found that female professional athletes carry a three times greater chance of experiencing urinary incontinence (UI) in comparison to non-active women.9 Due to the reactive nature and high demands of college athletics, college athletes are theoretically more susceptible to PFD and associated incontinence. Consequently, an overall prevalence of PFD has been reported to be seen in as high as 33% of athletes including 45% in females and 14.7% in males.5 Aside from the physical aspects of PFD, athletes may also experience embarrassment or anxiety commencing a domino effect that leads to a decline in athletic performance and overall quality of life (QoL).
As the research continues to amass regarding PFD, there are still many areas within the field that are severely understudied. To begin, a limited number of PFD studies include men as subjects. When men are included, the studies often feature a smaller number of male participants compared to females.9 This is concerning considering the similarities in clinical presentation between males and females. A second area lacking research is the impact PFD may have on an athlete’s performance and QoL. One meta-analysis found an association between UI and lower quality of life (QoL) scores.10 However, this study had limitations as it solely focused on UI without considering other PFD symptoms or diagnoses. Additionally, many studies included in the metaanalysis were at high risk of bias as individuals prone to developing UI are also at risk for comorbidities that might negatively impact QoL.10
The purpose of this study was to explore the prevalence of PFD among college-aged athletes, assess how PFD im-
pacted athletic performance, and identify contributing factors for increased likelihood of PFD in athletes.
In this cross-sectional study data were collected from participants through an in-person screening to provide a comprehensive overview of the characteristics and behaviors of the selected population. This approach was chosen to describe the current status of Lebanon Valley College (LVC) athletes rather than attempting to determine causation of PFD or provide information about changes over time. As typical in descriptive studies, a survey was deployed to collect demographic information and data on the prevalence of PFD The institutional review board (IRB) at LVC, IRB (#2023-12), approved this study.
Participants were recruited by convenience sampling through electronically messaging all twelve men’s and all thirteen women’s LVC NCAA Division 3 sports teams. Athletes were encouraged to attend one of three screening sessions that were being held throughout the course of one day at the LVC athletic Field. In order for a participant to be included in the study, they were required to be a current LVC student and a fully participating, NCAA Division 3 varsity team member. There were no restrictions on participant playing vs. bench time when determining inclusion criteria for this study. Individuals were excluded from the study if they were not a current LVC student, not a fully participating NCAA Division 3 varsity team member, or if they played a sport in a previous year of college but were not fully participating at the day/time screenings were completed. Informed consent was obtained at the beginning of the screening process.
The screening process had two parts. The first part served to gather demographic information and a Cozean Pelvic Dysfunction Screening Protocol (Cozean) score from all participants. Characteristics such as age, sex assigned at birth, self-identified gender, sport team participation, participant’s general knowledge of PFD/PFM (assessed on a 0-10 scale) were obtained via a demographic survey questionnaire. The Cozean score was obtained from administrating the Cozean survey The Cozean survey was established in 2018 by Nicole Cozean and Jesse Cozean and includes ten questions regarding signs and symptoms of PFD (Appendix A).11 If applicable, the participant checks the box next to the related statement and the sum of the total number of boxes checked equals a score (0-10). The Cozean was utilized in this study as there is a 91% specificity indicating possible PFD in individuals who score greater than or equal to 3 at the end of the survey.11
Due to the specificity noted above, the second part of the screening process was administered only to participants who scored 3 or greater on the Cozean. The second screening sought to obtain individual participant ratings, on a 0-10 scale, of the impact PFD had on their athletic performance and personal life and if the influence of PFD made
them feel embarrassed, anxious/worried, annoyed, and/or frustrated. This data was obtained from administration of an additional survey drafted by the investigators (Appendix B).
All participants who completed the second screening were then randomly assigned to one of three investigators to undergo a coccygeal motion palpation (CMP) assessment. These three investigators were trained by a Certified Pelvic Rehabilitation Practitioner and an American Board of Physical Therapy Specialties Certified Women’s Health Specialist physical therapist to identify the contraction, release, and lengthening of pelvic floor muscles through this objective, external, and noninvasive assessment. The CMP is a valid screening assessment that has been previously found to have a 94% sensitivity and 79% specificity in identifying underactive, overactive, or normal PFM activity 12 To begin the CMP, participants were placed in a seated position. Next, external palpation of the PFM occurred by the investigator placing the palmar side of their hand over the base of the participant’s sacrum and informing the participant that the tip of their coccyx would be palpated by the investigator’s finger tip. Following this, participants were verbally instructed to initially contract, then bear down, and then to do nothing to their PFMs. As the participant completed each command, the investigator determined whether the participant was presenting with underactive, overactive, or normal PFM activity. Overactive PFM activity was determined if minimal movement with contraction and lengthening occurred due to the muscles being in a hypertonic state. Underactive PFM activity was determined if increased movement with contraction and lengthening occurred due to the muscles being in a hypotonic state. Normal PFM activity was determined if no indications of a hypertonic or hypotonic state were present. It is important to note that all determinations of participant PFM activity by the three investigators were confirmed from a second CMP assessment completed by the Certified Pelvic Rehabilitation Practitioner and an American Board of Physical Therapy Specialties Certified Women’s Health Specialist physical therapist. All assessors were in agreement with the determinations of participant PFM activity to be used for scoring and comparisons.
Data were transferred from paper format into IBM Statistical Package for the Social Sciences (SPSS) version 28 for analysis. In order to examine the results thoroughly, the data were analyzed in two different subgroups. The first subgroup included all 53 participants, and the second only included participants who scored 3 or more on the Cozean. Data of all 53 cases were examined for normalcy and outliers prior to completing analyses. For both subgroups, Pearson correlation coefficients were calculated for the relationship between participant’s age and Cozean score and a participant’s indicated knowledge of PFD and Cozean score. Additionally, an independent-samples t test was calculated comparing the mean Cozean scores of those who identified themselves as male and those who identified themselves as female, and a one-way ANOVA comparing participants’ Cozean score and their current sport involvement (swimming, football, and other) were calculated. Ef-
fect sizes for statistically significant outputs were determined by computing the coefficient of determination (r2) for Pearson correlation analyses (r2 values < 0.25 and ≥ 0.09 indicate a moderate effect), Cohen’s D for independent-samples t test (≥ 0.80 indicates a large effect), and eta squared (η2) for one-way ANOVA analysis (η2 values < 0.25 and ≥ 0.09 indicate a moderate effect).13 The hypotheses were tested based on the statistical significance criteria of a pre-established (a priori) probability alpha (α) level of α = .05.
The age range of participants was between 18-25 years-old. All participants individually responded that their sex assigned at birth and identified gender were identical. Therefore, the participants were composed of 37 males and 17 females. Participants identified they were currently playing the following sports: football (31), swimming (14), track and field and cross country (3), dance (2), tennis (1), golf (1), and soccer (1). Due to the decreased number of participants in sports, the demographic variable of sport was categorized into three main groups; football, swimming, and other The average of participant knowledge of the pelvic floor was 2.68/10. Lastly, 24.5% of the participants scored ≥ 3 on the Cozean. Table 1 outlines participant demographics.
Concerning the first subgroup, a moderate positive correlation was found (r(51) = .391, p < 0.001), indicating a significant relationship between age and Cozean score (Table 2). Older participants tended to score higher on the Cozean. The effect size (r2) is 0.153, indicating a moderate effect.
A significant difference in Cozean scores between the males and females was found (t(51) = -3.959, p < 0.05) (Table 3). The effect size was calculated for this analysis and found to be large (d = 1.81).
A significant difference in Cozean scores was found among the sport categories (F(2, 50) = 8.288, p <0.001). A moderate effect size for the Analysis of Variance model was calculated (η2 = 0.249). Tukey’s HSD was used to determine the nature of the differences between the sports. This analysis revealed that participants who played football scored lower (M = 1.29, sd 0.82) than participants who played swimming (M = 2.86, sd 1.61), indicating a higher frequency of PFD symptoms in swimming athletes as compared to football athletes. Participants who played all other sports (M = 2.5, sd 2.0) were not significantly different from either of the other two groups (Table 4).
A moderate positive correlation was found (r (51) = .431, p < 0.001), indicating a significant relationship between participants who indicated increased knowledge of PFD and Cozean score. The effect size (r2) is 0.186 indicating a moderate effect.
For the second subgroup, 13 out of the 53 participants (25%) scored 3 or greater on Cozean. Of the 13, 53.8% had underactive PFM and 38.5% had overactive PFM, as assessed by the CMP, for a total of 92.3% of the second subgroup population. No statistical significance was found amongst the demographic variables with the second sub-
Table 1. Participant Demographics N = 53
Cozean Score
group of participant analysis. Of this subgroup, 69% felt embarrassed, anxious/worried, and annoyed, while 61.5% felt frustrated. In addition, 69% reported that PFD had a discernible effect on their athletic performance and 77% indicating a negative impact on their personal life.
Data analysis of the subgroup including all 53 participants showed that the factors of age, sex, sport, and self-knowledge were significant in a participant scoring ≥ 3 on the Cozean. Despite the participant age range of 18 to 25, there was still a notable difference in older participants exhibiting higher scores on the screening tool. These findings
highlight and advance the importance of age as a significant factor in predicting PFD 14 For healthcare clinicians, this relationship has practical implications when conducting pelvic dysfunction screenings on college-aged athletes. Previous research has linked increased age to pelvic floor dysfunction in both males and females.15 This finding demonstrates that trends continue to exist in younger populations.
In this study, gender differences did impact Cozean scores. This was consistent with prior research as it highlights that gender must be considered in the context of PFD screening.16 The large effect size suggests that this distinction is not due to chance. It is important to note that this study was able to identify this difference when the number of male participants was double the number of females. This factor helps to fill a major gap in PFD research in a male population.17,18 Medical providers should be aware of these gender-based differences when evaluating and addressing PFD in athletes.
A key element identified in this study was the impact an athlete’s sport may have on their PFM. The analysis revealed that participants who played football scored significantly lower on the Cozean compared to those who participated in swimming. This result suggests that not all
Table 3. Gender Independent-Sample t Test Statistics
Levene’s Test for Equality of Variances
Table 4. ANOVA Statistics for Sport
sports have an equal impact on Cozean scores indicating that physical activity and demand of sport plays a factor in PFM health.19,20 These results necessitate further research into the specific factors within each sport that may influence PFD Such research could have implications for coaching and athlete development, as well as interventions aimed at raising awareness of symptoms of PFD dysfunction within the sporting community.
Another key element in the first subgroup of data analysis was the significant relationship between a participants’ self-indicated knowledge of PFD and their Cozean score. This moderate effect size suggests that those who have greater knowledge of the pelvic floor tended to achieve higher scores on the Cozean. This is consistent with a prior study that investigated the impact of education and knowledge on symptoms of PFD 4 These results underscore the critical role of patient education and awareness in the context of pelvic dysfunction. By healthcare providers and clinicians providing education to patients, they are more likely to self-identify symptoms.21 This may lead to earlier detection and management of pelvic dysfunction should it be present.22 By equipping providers with the tools to educate patients, there is an aim to facilitate early symptom recognition and encourage individuals to seek medical assistance at an earlier stage and mitigating the development of chronic issues.
Analysis in the second subgroup concluded that 12 of the 13 participants who scored ≥ 3 on the Cozean had an over/under active PFM on external palpatory confirmation testing. This indicates that the Cozean screening tool accurately identified those at an increased likelihood of having PFD, achieving a 92.3% accuracy rate in identifying those with PFD based on the 10 screening tool questions.11 With the Cozean having a 91% specificity, the current data reinforces the screening tool’s effectiveness in correctly identifying individuals with conclusive PFD among those who scored ≥ 3.11
In regards to the 13 individuals who scored ≥ 3 on the Cozean, no significant difference between demographic variables and Cozean scores was found. This could indicate that regardless of gender, sport, age, and self-knowledge of PFD, individuals experience a similar impact of symptoms or likelihood factors associated with PFD However, it is im-
portant to note that while impact is similar, the prevalence may vary
Aside from physical symptoms, athletes with PFD experience a strong psychological component thus impacting their mental health.23 These effects were truly self-determined, as at that time during the screening process, there was no hands-on evidence to provide determination of PFD Based on these results, there is evidently a strong psychosocial factor that has a negative impact on quality of life. From the athlete’s perspective, negative feelings about their symptoms may lead to a poor societal image, diverting attention away from their athletic performance, which was also shown to be impacted by the results of this study.
The above findings can be very beneficial to understand with application to future athletic screenings. Factors such as age, sex, sport, and self-knowledge must be considered, as these were found to be related to Cozean scores. If athletes experience symptoms of PFD, it can be reassuring for them to know that this is not uncommon and that deficits can be addressed with pelvic floor interventions.24 As noted, addressing the psychological component of PFD is vital in ensuring that coaches/trainers are advocating for mental health. This reassurance could decrease anxiety or fear of being socially outcasted due to PFD.
Limitations to this study which should be considered when interpreting the results include the method of convenience sampling, potential reporting bias, and lack of private setting for external examination. A convenience sampling method was used due to time and geographic constraints. As a result, the sample is not representative of the entire population of collegiate athletes (only Division III athletes) and generalizability may be diminished. The recruitment method led to an unequal distribution of participants across the various sports studied, and some sports were not included at all, further limiting generalizability The validated Cozean survey used to collect data included questions of a private nature related to symptoms of PFD. The inherently sensitive nature of these questions may have led to underreporting or misrepresentation of symptoms due to social or self-image pressures. This potential bias could impact the accuracy and completeness of the data collected. Finally, the absence of a private setting during the data collection process may have contributed
to the potential reporting bias mentioned above. Although this is the standard of care, a private setting for the external palpation was unavailable for the completion of this study As a result, participants may have felt uncomfortable, distracted, self-conscious, anxious, stressed, or embarrassed when providing their responses which could have influenced the accuracy of the data. By ensuring a more private, confidential, supportive, and safe treatment/assessment area may lead to more honest and accurate responses. This study has highlighted the need for future research regarding PFD and how it affects college-aged athletes. As topics surrounding the PFM are sensitive in nature, future screenings, similar to the assessments completed in this study, should be conducted in a private setting. Utilizing separate rooms or using curtains would improve this investigation by increasing privacy to help ensure that participants feel comfortable and relaxed. This would enhance the accuracy of participant responses. Additionally, an increase in the number and variety of participants in a similar study would amplify the findings and improve upon generalizing results. Lastly, while personalized treatments, education, and interventions used to treat PFD are already known to be effective, a future study could assess the impact of a gener-
alized recommendation of 3-4 exercises for the broad categories of either underactive or overactive PFM.
The results of the current study demonstrated that older female NCAA Division III college athletes who participate in swimming and who possess self-knowledge of PFD are more likely to experience PFD. Additionally, these athletes are likely to encounter a significant impact on their athletic performance and quality of life. These results provide preliminary evidence on the need of PFD awareness and assessment among college athletes.
The authors report no conflicts of interest.
Submitted: March 13, 2024 CDT, Accepted: June 10, 2024 CDT
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Tim S, Mazur-Bialy AI. The most common functional disorders and factors affecting female pelvic floor. Life (Basel). 2021;11(12):1397. doi:10.3390/life11121397
2. Betschart C, Singer A, Scheiner D Beckenboden der Frau: Anatomie und normale Funktion [Female pelvic floor: anatomy and normal function]. Ther Umsch 2019;73(9):529-534. doi:10.1024/0040-5930/ a001035
3. Wallace SL, Miller LD, Mishra K. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019;31(6):485-493. doi:10.1097/ GCO.0000000000000584
4. Berzuk K, Shay B. Effect of increasing awareness of pelvic floor muscle function on pelvic floor dysfunction: a randomized controlled trial. Int Urogynecol J. 2015;26(6):837-844. doi:10.1007/ s00192-014-2599-z
5. Culleton-Quinn E, Bø K, Fleming N, et al. Elite female athletes’ experiences of symptoms of pelvic floor dysfunction: A systematic review Int Urogynecol J 2022;33(10):2681-2711. doi:10.1007/ s00192-022-05302-6
6. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: A randomized clinical trial. J Sex Marital Ther. 2019;45(5):378-394. doi:10.1080/ 0092623X.2018.1549631
7 Lawson S, Sacks A. Pelvic floor physical therapy and women’s health promotion. J Midwifery Womens Health 2018;63(4):410-417 doi:10.1111/jmwh.12736
8. Rebullido TR, Gómez-Tomás C, Faigenbaum AD, Chulvi-Medrano I. The prevalence of urinary incontinence among adolescent female athletes: A systematic review J Funct Morphol Kinesiol 2021;6(1):12. doi:10.3390/jfmk6010012
9. Rodríguez-López ES, Calvo-Moreno SO, BasasGarcía Á, et al. Prevalence of urinary incontinence among elite athletes of both sexes. J Sci Med Sport. 2021;24(4):338-344. doi:10.1016/j.jsams.2020.09.017
10. Pizzol D, Demurtas J, Celotto S, et al. Urinary incontinence and quality of life: A systematic review and meta-analysis. Aging Clin Exp Res 2021;33(1):25-35. doi:10.1007/s40520-020-01712-y
11. Cozean N. Simple screening questionnaire for pelvic floor dysfunction. Herman and Wallace. Published October 2018. Accessed February 6, 2023. https://hermanwallace.com/blog/simple-screeningquestionnaire-for-pelvic-floor-dysfunction
12. Maher RM, Iberle J. Concurrent validity of noninvasive coccygeal motion palpation and transabdominal ultrasound imaging in the assessment of pelvic floor function in women. J Wom Health Phys Ther 2020;44(4):176-181. doi:10.1097/ jwh.0000000000000175
13. Cronk BC. How to Use SPSS. A Step-by-Step Guide to Analysis and Interpretation 10th ed. Routledge; 2018. doi:10.4324/9781315142999
14. MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. Int J OB GYN. 2000;107(12):1460-1470. doi:10.1111/j.1471-0528.2000.tb11669.x
15. Burnett LA, Cook M, Shah S, et al. Age-associated changes in the mechanical properties of human cadaveric pelvic floor muscles. J Biomech 2020;98:109436. doi:10.1016/j.jbiomech.2019.109436
16. Khowailed IA, Disney H, Lee H. Gender-specific differences of normative values of pelvic floor muscle function in healthy adults population: An observational analytical study. Women Health. 2020;60(10):1185-1195. doi:10.1080/ 03630242.2020.1807449
17 Giagio S, Salvioli S, Pillastrini P, Innocenti T Sport and pelvic floor dysfunction in male and female athletes: A scoping review Neurourol Urodyn 2021;40(1):55-64. doi:10.1002/nau.24564
18. Knol-de Vries GE, Malmberg GGA, NotenboomNas FJM, et al. Exploring concomitant pelvic floor symptoms in community-dwelling females and males. Neurourol Urodyn. 2022;41(8):1770-1780. doi:10.1002/nau.25020
19. Bø K, Nygaard IE. Is physical activity good or bad for the female pelvic floor? A narrative review. Sports Med 2020;50(3):471-484. doi:10.1007/ s40279-019-01243-1
20. Dakic JG, Hay-Smith J, Lin KY, Cook J, Frawley HC. Experience of playing sport or exercising for women with pelvic floor symptoms: A qualitative study. Sports Med Open. 2023;9(1):25. doi:10.1186/ s40798-023-00565-9
21. Fernandes ACNL, Palacios-Ceña D, Hay-Smith J, et al. Women report sustained benefits from attending group-based education about pelvic floor muscles: A longitudinal qualitative study. J Physiother 2021;67(3):210-216. doi:10.1016/ j.jphys.2021.06.010
22. Goodridge SD, Chisholm LP, Heft J, et al. Association of knowledge and presence of pelvic floor disorders and participation in pelvic floor exercises: A cross-sectional study. Female Pelvic Med Reconstr Surg 2021;27(5):310-314. doi:10.1097/ SPV.0000000000000813
23. Larouche M, Brotto LA, Koenig NA, et al. Depression, anxiety, and pelvic floor symptoms before and after surgery for pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2020;26(1):67-72. doi:10.1097/SPV.0000000000000582
24. Al-Badr A, Saleem Z, Kaddour O, et al. Correction to: Prevalence of pelvic floor dysfunction: A Saudi national survey. BMC Womens Health. 2023;23(1):274. doi:10.1186/s12905-023-02432-x
Download: https://ijspt.scholasticahq.com/article/120211-screening-for-incidence-and-effect-of-pelvic-floordysfunction-in-college-aged-athletes/attachment/232839.docx?auth_token=F2r3yAIHEJjF-mRtqtda
Download: https://ijspt.scholasticahq.com/article/120211-screening-for-incidence-and-effect-of-pelvic-floordysfunction-in-college-aged-athletes/attachment/232838.docx?auth_token=F2r3yAIHEJjF-mRtqtda

Patterson RD, Zettlemoyer A, Plackowski M, Baker R, Cheatham SW, Nasypany A. The Effects of TMR® Fab 6 on Hamstring Flexibility in Healthy Subjects; An Exploratory Observational Investigation. IJSPT. Published online July 1, 2024:877-887. doi:10.26603/001c.120203
Richard D. Patterson1a , Alexander Zettlemoyer2 , Mary Plackowski3 , Russell Baker4 , Scott W. Cheatham5 , Alan Nasypany4
1 Exercise, Health, & Sport Sciences, Pennsylvania Western University , 2 Mechanicsburg Area School District, 3 Health Sciences and Human Movement, Colorado State University Pueblo, 4 Movement Sciences, University of Idaho, 5 Kinesiology, California State University, Dominguez Hills
Keywords: Hamstring flexibility, Hamstring extensibility, Movement asymmetry, Musculoskeletal dysfunction
https://doi.org/10.26603/001c.120203
Background
Stretching programs are designed to improve hamstring flexibility by attempting to mechanically increase the length of the target tissue. However, other manual treatment approaches such as those utilized in Total Motion Release (TMR®), could be beneficial by identifying body asymmetries to assess and treat soft tissue impairments leading to diminished extensibility
Purpose
The purpose of this study was to determine the effectiveness of the TMR® Fab 6 assessment and treatment to increase hamstring flexibility in healthy participants following one session of TMR®.
Observational Cohort study
Methods
A convenience sample of 20 healthy participants (10 males, 10 females) were recruited from three institutions. Following collection of demographic information and a brief medical history, each participant performed a five minute warm-up on the stationary bike at a moderate intensity (80-90 RPMs) followed immediately by the bilateral performance of the Active Knee Extension Test (AKET) and Passive Straight Leg Raise (PSLR) to assess hamstring muscle length. Participants were randomly placed in the TMR® or control group. The TMR® group completed the “Fab 6” evaluation and treatment, while the control group performed one repetition of standing active hip flexion every 30-seconds for 15-minutes with both knees in full extension. Upon completion of treatment, control and TMR® groups were immediately re-evaluated on the AKET and the PSLR in the same order and fashion as baseline testing. Participants were asked to return in 24-hours for the same objective measurements as previously described.
Results
A significant time by group interaction was identified across all variables (p ≤ 0.001) for AKET and PSLR except the PSLR preferred leg from post-treatment to 24hr follow-up. The most significant increase in the AKET occurred in the TMR® group between baseline and post-treatment of the non-preferred leg (12.15°±2.94) when compared to the control group (7.15°±1.56).
Corresponding Author:
Rpatterson@pennwest.edu a
Rich D. Patterson DAT, LAT, ATC
Department of Exercise, Health & Sport Sciences
Pennsylvania Western University California, PA 15419
724-504-3307
The results of the study suggest that implementing a regionally interdependent treatment approach like TMR® results in significant improvements in hamstring extensibility and hip ROM compared to the control group.
Level of evidence 3
Stretching programs typically focus on mechanically increasing the extensibility of the target tissue through deformation (i.e., plastic and viscoelastic), increased sarcomeres in series, neuromuscular relaxation, or modification of sensory perception.1 The use of active and passive stretching techniques may increase the stretch tolerance in patients rather than fostering a physiological change in the mechanical properties of the muscle.1‑3 Despite the lack of evidence to support a permanent deformation of musculoskeletal tissue as a result of progressive stretching, clinicians frequently incorporate stretching as part of an injury prevention or rehabilitation protocol.4‑7
The application of Proprioceptive Neuromuscular Facilitation (PNF), Static Stretching (SS), and Dynamic Stretching (DS) techniques are thought to influence the target tissue (e.g., hamstring musculature) extensibility. However, these techniques produce little effect on the musculoskeletal soft tissue restriction as the interventions may negatively influence adjacent structures or neighboring joint mobility. In the hamstring, for example, stretching programs designed to optimize muscle lengthening could alter or inhibit adjacent structures, such as lumbar or pelvic postural stabilizers, resulting in a perceived change in hamstring length.8 As a result, stretching protocols may produce limited mechanical lengthening at the site of tissue restriction, but instead increase range of motion (ROM) through a series of postural and motor control changes up and down the kinetic chain. Long-term stretching protocols have been shown to produce suboptimal movement patterns, structural malalignment and inhibit neuromuscular control, which develop in response to musculoskeletal imbalances and localized tissue adaptions.9‑11
Conflicting evidence suggests that short-term or acute changes in hamstring extensibility using traditional stretching techniques are achievable but physiological elongation of the target tissue is unrealistic.3,4,12‑15 Focusing on impairments from other body regions or systems, regardless of the proximity to the musculoskeletal dysfunction, rather than treating with a local intervention targeting the perceived impaired tissue produces a greater impact on the patient’s primary complaint.8,9 A novel treatment approach, such as Total Motion Release® (TMR®), which attempts to restore mobility of surrounding structures and reduce adjacent soft tissue restriction has demonstrated greater benefits than traditional stretching for improving ROM. Restoring movement symmetry of surrounding structures that influence target tissue to improve joint mobility, such as in a regional interdependence

approach, could produce long-lasting benefits in restricted tissue.
TMR® is a manual treatment technique developed by Tom Dalonzo-Baker which identifies body asymmetries to assess and treat dysfunction.16 The technique uses a regional interdependence approach to increase mobility and decrease pain and restriction within the musculoskeletal system.17 The Level one TMR® treatment technique includes a movement assessment which incorporates six bilateral movements (referred to as the “Fab 6”) performed across the upper body, trunk, and lower body (Figure 1).18 Following each movement, the patient rates the motion on a subjective scale from 1 (no dysfunction, pain, asymmetry) to 100 (complete dysfunction, pain, asymmetry).19 The upper body, trunk, and lower body motions with the greatest dysfunction are treated by repeating the TMR® Fab 6 movement using the patient’s “good side.” Recent research suggests that TMR® is effective in treating soft-tissue restriction of both the upper and lower extremities. Gamma et al19,20 demonstrated significant increases in shoulder internal and external rotation of healthy baseball pitchers using the TMR® trunk-twist and arm raise movements compared to a traditional static and dynamic warm-up. Baker et al.16 also demonstrated a significant increase of 31.5° bilaterally in the active straight leg raise (ASLR) following one week of TMR® combined with instrument assisted soft-tissue mobilization.
To date, no studies evaluating the efficacy of the TMR® Fab 6 protocol on lower extremity hamstring muscle extensibility could be identified. Therefore, the purpose of this exploratory observational study was to determine the effectiveness of the TMR® Fab 6 assessment and treatment to increase hamstring flexibility in healthy participants following one session of TMR®. The authors hypothesized that restoring movement symmetry utilizing the TMR® Fab 6 would result in a greater increase in hamstring flexibility as measured by the Active Knee Extension Test (AKET) and
the Passive Straight Leg Raise Test (PSLR) when compared to a control group.
An observational study approach was used to evaluate independent group variables (i.e., TMR and control) and limb (i.e., preferred leg and non-preferred leg). The non-preferred leg was considered the extremity with the least amount of flexibility at baseline while the preferred leg was considered the extremity with the greatest amount of flexibility at baseline. This protocol was determined to be most appropriate because TMR® is designed to treat movement asymmetry throughout the body rather than target a specific tissue dysfunction. The terms “preferred” and “ nonpreferred” were used to avoid the need to operationalize dominance because, unlike the upper extremity, there is no consensus when defining lower extremity dominance. In some cases, lower extremity dominance is determined by asking the patient which leg they prefer to kick a ball.21 However, this definition assumes that the limb used to kick a ball would be the same as the limb with greatest strength, the limb used to “brake” after being pushed, the limb used to jump, and the limb used to spontaneously land following a step-up task.22 In addition, there has been no association found between self-identified preferred kicking leg and preferred landing leg in athletes and non-athletes, therefore, lower extremity dominance has no impact when analyzing movement asymmetries.21,23,24 The dependent variables were AKET range of motion and PSLR range of motion at baseline, post treatment, and 24 hour follow up.
A convenience sample of 20 healthy participants were recruited from three separate institutions (two colleges, one high school). All participants were high school or college athletes who remained physically active but had not engaged in an organized team or individual sport within the previous six months. Participants were randomly assigned to either the TMR® group or the control group. Participants were excluded from the study if they had a history of hamstring pathology that prevented full knee or hip range of motion, a lumbo-pelvic pathology limitation which prohibited participation in the PSLR, ongoing musculoskeletal, neuromuscular or functional limitations, history of paresthesia, motor abnormality, a knee flexion angle greater than 70 degrees while performing the AKET, or if the participant was involved in a systematic stretching program within the last month. The institutional review board from each facility approved this study in the spirit of the Helsinki Declaration, and all participants provided written informed consent including minor participant assent and parental consent. Prior to baseline evaluation, all participants were asked to refrain from pre-activity warm-up or stretching within the two hours preceding assessment and treatment.
TOTAL MOTION RELEASE® FAB 6 TREATMENT GROUP
The TMR® treatment group subjectively compared the Fab 6 movements bilaterally to determine the side and/or motion of greatest restriction. The six movements include: seated shoulder flexion or arm raise (AR), bent arm wall push-up or arm press (AP), seated trunk rotation (TR), seated hip flexion with knee extension or leg raise (LR), single leg sit to stand (STS) and unilateral standing toe touch (TT).18 Following each movement, a visual scoring scale (Table 1) was used to identify the “good side” (lower score) and “bad side” (higher score) related to participant reported pain, tightness, ROM, strength, and ease of motion. The most asymmetrical upper body movement (i.e., shoulder flexion or wall push-up), lower body movement (i.e., straight leg raise, toe touch, or sit-to-stand) and trunk movement (i.e., rotation right or rotation left) were selected and treated. A standard provocative movement (e.g., standing hip flexion) was also established to assess patient reported subjective hamstring restriction. The standard provocative movement was utilized throughout the testing procedure to assess the impact of the TMR® Fab 6 treatment on perceived hamstring tightness.
The control group performed the same baseline testing and standard provocative movement (standing hip flexion). Following intake and ROM measurements, participants were instructed to perform one repetition of standing active hip flexion with both knees in full extension, held for 2 seconds and repeated every 30 seconds for 15 minutes. The sham treatment was created to ensure a temperature increase in the target tissue and provide results similar to a dynamic warm-up.
Active Knee Extension Test. The AKET was performed with the participant in a supine position inside a homemade AKET device (Figure 2) with the non-test leg secured to the table in a pelvic neutral position using a mobilization belt across the mid-portion of the anterior thigh. The TiltMeter smartphone app (Carlos Hernandez) was placed on the participant’s anterior thigh, 10 cm proximal to the superior pole of the patella of the tested leg. The participant’s thigh was passively flexed to 90 degrees and maintained in that position using the horizontal bar of the homemade AKET device.25 The participant was then asked to actively extend the knee until reaching the maximal tolerable stretch of the hamstring muscles.26,27 The examiner placed the TiltMeter smartphone app on the anterior tibia halfway between the inferior pole of the patella and distal tibia with the screen facing away from midline to blind the participant.26 Measurements were recorded as the distance from 180 degrees of knee extension. Prior to data collection, interrater reliability was established using a sample of 11 healthy participants independently recruited for this por-
Table 1. Visual scoring scale for TMR®
tion of the study Interrater reliability assessment for AKET and PSLR was assessed using a two-way mixed intraclass correlation coefficient (ICC3,1). Active knee extension testing was reliable between raters for the right leg (ICC3,1 = 0.952, p<.0001, 95% CI: 0.90, 0.99, SEM 3.32) and the left leg AKET (ICC3,1 = 0.97 (p<0.001, 95% CI: 0.93, 0.99, SEM 3.06) for the three raters. Interrater reliability results are consistent with other researchers who found an ICC2,1 value of 0.93 for the AKET.28 Reliability for each rater was also assessed, and also indicated excellent intra-rater reliability with values ranging from 0.94-0.97 (p<.0001, 95%CI: 0.74, 0.99). Standard error of measurement (SEM=SD* √ 1-test reliability) was calculated for test-retest reliability of the AKET with measurements ranging from 1.50-3.72.
Passive Straight Leg Raise Test. Hip flexion was assessed bilaterally using the PSLR test. The participant was positioned supine with both legs extended and the non-test leg secured to the table in a pelvic neutral position using a mobilization belt across the mid-portion of the anterior
thigh. Participants were also instructed to relax the ankle in a slightly plantarflexed position.25 The TiltMeter smartphone app was placed on the anterior tibia halfway between the inferior pole of the patella and the distal tibia with the screen facing away from the midline to blind the participant. The hip was passively flexed until the participant identified a strong but tolerable stretch in the hamstring and measurements were recorded. A reliability testing session revealed that interrater reliability for the passive straight leg raise test was excellent for the right leg (ICC3,1 = 0.97, p<0.001, 95%CI: 0.93, 0.99, SEM 2.58) and the left leg PSLR (ICC3,1 = 0.98, p<0.001, 95% CI: 0.96, 0.99, SEM 2.27), which was consistent with the existing literature.28 Intrarater reliability was found to be 0.94-0.97 (p<.0001, 95%CI: 0.70, 0.99) for the PSLR across the three raters. Lastly, standard error of measurement was calculated for PSLR test-retest reliability, which ranged from 1.74-3.45.
The TMR® treatment group utilized the “good side,” performing the unilateral motion described during the FAB 6 evaluation. Of the three previously identified body regions (e.g., upper body, lower body and trunk twist), the most asymmetrical movement was performed first by having the participant complete two sets of 10 repetitions (one round) of an isotonic motion to the end range. For example, if shoulder flexion was considered the most asymmetrical motion, the participant performed 2 sets of 10 repetitions of shoulder flexion to the end range using the “good side” with 30-seconds rest between sets. Following the first round, the participant was instructed to retest the same movement (e.g., shoulder flexion) on the “bad side” and re-score on the visual scoring scale. At this time, the participant also retested and rescored bilateral standing hip flexion (provocative motion) to establish if the previously identified hamstring restriction had changed. Traditional TMR® protocol governed the progression of TMR® following round one, but the rules were modified for subsequent rounds to establish consistency across three examiners (Table 2). Rounds of the identified TMR® movement continued based on the the established rules matrix until the
Table 2. Modified rules matrix for TMR® treatment
Round 1 If TMR# improves (gets better), demonstrates little to no change (<10 points) or TMR# increases (gets worse) – Do same exercise again in the exact same way
Round 2 If TMR# improves (gets better) – Do same exercise again in the exact same way If TMR# has little to no change (<10 points) or TMR# increases (gets worse) – Switch to next greatest asymmetry for that body region
Round 3 If TMR# improves (gets better) – Do same exercise again in the exact same way If TMR# has little to no change (<10 points) or TMR# increases (gets worse) – Switch to next greatest asymmetry for that body region
Once the TMR# has been reduced to 5 or less – move on to the next exercise.
participant identified a 5 or less on the visual scoring scale before moving on to the next body region. This process continued until a score of 5 or less was recorded in all three body regions.
Participant intake and baseline evaluation were completed and included a medical history to verify exclusion criteria along with demographic information. After intake, each participant performed a 5-minute warm-up on the stationary bike at a moderate intensity (80-90 RPMs) followed immediately by the bilateral ROM evaluations on the right and left lower extremities in order from AKET to PSLR. Following baseline measurements, participants were randomly placed in the TMR® group or the control group. Upon completion of treatment, control and TMR® intervention groups were immediately re-evaluated using the AKET and the PSLR in the same order and fashion as baseline testing. Participants were asked to return in 24-hours for the same objective measurements as previously described. All participants were instructed to refrain from supplemental stretching and physical activity in which the heart rate was elevated for more than 15-minutes over the 24-hour period.
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS, Inc., Armonk, NY, USA) version 23.0. The results are expressed as mean ± standard deviation. Normality of distribution and equality of variance were assessed using Levene’s test. An independent samples t-test was used to assess group differences at baseline on the AKET and PLSR. A mixed-model ANOVA, with one between (group) and one within (time) factor, was used to determine any main effects, using the multivariate criterion of Wilks’ Lambda (λ), or interactions across time for each outcome measure across groups. The alpha level was set a priori at p< 0.05 for all analyses. If a significant ANOVA was found, an independent t-test was used to compare the changes between groups across the time points (e.g., baseline to post-intervention, post-intervention to 24hr follow-up, and baseline to 24hr follow-up). Post-hoc t-tests were corrected for type I error using the Bonferroni technique (αadjusted = .05/3) and the adjusted alpha level
was set a priori at p ≤ .017. Effect size was calculated using Cohen’s d ([M1 – M2] /standard deviation(pooled)) and a large effect size was set a priori at d ≥ .08.29
PRELIMINARY DATA ANALYSIS
A total of 20 participants (10 males and 10 females, mean age = 17.8±1.2 years, mean height = 175.0±10.2 cm, mean weight = 74.5±17.1 kg) were included, all met study criteria and completed all testing portions of the study. Significant differences between groups were not found on the demographic variables after group allocation (Table 3). Significant group differences between groups were also not found on baseline performances of the AKET or PSLR on either the PL or NPL (Table 3). ROM across all three timepoints between groups can be found in Table 4
ACTIVE KNEE EXTENSION TEST ANALYSIS OF THE NONPREFERRED LEG
A significant difference for time (F2,17 = 179.91, p ≤ 0.001, partialƞ2 = .955, power = 1.0) and time by group interaction (F2,17 = 57.38, p ≤ 0.001, partialƞ2 = .871, power = 1.0) were found in the AKET on the non-preferred leg. Immediately following the intervention, the TMR® group (mean change = 12.15° ± 2.94) experienced an improvement on the AKET that was significantly better (mean difference = 5.00°, p ≤ 0.001, Cohen’s d = 2.12, 95% CI 2.78, 7.22) than the improvement experienced by the control group (mean change = 7.15° ± 1.56). From the post-treatment measure to the 24hr follow-up measure, the TMR group (mean change = -2.85° ± 1.36) maintained significantly more (mean difference = 3.90°, p ≤ 0.001, Cohen’s d = 2.74, 95% CI 0.63, 2.57) of the improvement than the control group (mean change = -6.75° ± 1.48). From the baseline measure to the 24hr follow-up measure, the TMR group (mean change = 9.30° ± 2.10) displayed significantly improved (mean difference = 8.90°, p ≤ 0.001, Cohen’s d = 4.36, 95% CI 6.98, 10.81) AKET values compared to the control group (mean change = 0.40° ± 1.98).
Table 3. Mean differences between TMR and control groups at baseline (P>.05)
Table 4. Range of motion measures across time.
ACTIVE KNEE EXTENSION TEST ANALYSIS OF THE PREFERRED LEG
A significant difference for time (F2,17 = 154.60, p ≤ 0.001, partialƞ2 = .948, power = 1.0) and time by group interaction (F2,17 = 20.14, p ≤ 0.001, partialƞ2 = .703, power = 1.0) were found in the AKET on the PL. Immediately following the intervention, the TMR group (mean change 12.15° ± 3.05) experienced an improvement on the AKET that was significantly better (mean difference 4.15°, p ≤ 0.002, Cohen’s d = 1.61, 95% CI 1.72, 6.57) than the improvement experienced by the control group (mean change = 8.00° ± 2.00). From post-treatment measure to 24hr follow-up measure, the TMR group (mean change = -3.45° ± 1.61) maintained significantly more (mean difference = 4.45 p ≤ 0.001, Cohen’s d = 1.77, 95% CI 2.09, 6.81) of the improvement than the control group (man change = -7.90° ± 3.16). From baseline measure to 24hr follow-up measure, the TMR group (mean change = 8.70° ± 3.62) displayed significantly improved (mean difference = 8.60°, p ≤ 0.001, Cohen’s d = 2.94, 95% CI 5.82, 11.37) AKET values compared to the control group (mean change = 0.10° ± 2.07).
PASSIVE STRAIGHT LEG RAISE ANALYSIS OF THE NONPREFERRED LEG
A significant difference for time (F2,17 = 112.86, p ≤ 0.001, partialƞ2 = .930, power = 1.0) and time by group interaction (F2,17 = 33.21, p ≤ 0.001, partialƞ2 = .797, power = 1.0) were found in the PSLR on the non-preferred leg. Immediately following the intervention, the TMR group (mean change = 12.95° ± 3.10) experienced an improvement on the PSLR that was significantly better (mean difference = 6.95°, p ≤ 0.001, Cohen’s d = 2.53, 95% CI 4.37, 9.53) than the improvement experienced by the control group (mean change = 6.00° ± 2.33). From the post-treatment measure to the 24hr follow-up measure, the TMR group (mean change = -2.70° ± 1.03) maintained significantly more (mean difference = 2.35°, p ≤ 0.001, Cohen’s d = 1.09, 95% CI 0.32, 4.37) of the improvement than the control group (mean change = -5.05° ± 2.87). From the baseline measure to the 24hr follow-up measure, the TMR group (mean change = 10.25° ± 2.97) displayed significantly improved (mean difference = 9.30, p ≤ 0.001, Cohen’s d = 3.76, 95% CI 6.97, 11.62) PSLR values compared to the control group (mean change = 0.95° ± 1.85).
PASSIVE STRAIGHT LEG RAISE ANALYSIS OF THE PREFERRED LEG
A significant difference for time (F2,17 = 85.11, p ≤ 0.001, partialƞ2 = .909, power = 1.0) and time by group interaction (F2,17 = 27.11, p ≤ 0.001, partialƞ2 = .761, power = 1.0) were found in the PSLR on the PL. Immediately following the intervention, the TMR group (mean change = 13.70° ± 3.38) experienced an improvement on the PSLR that was significantly better (mean difference = 8.60°, p ≤ 0.001, Cohen’s d = 2.70, 95% CI 5.60, 11.60) than the improvement experienced by the control group (mean change = 5.10° ± 2.98). From post-treatment measure to 24hr follow-up measure, the TMR group (mean change = -3.80° ± 2.31) maintained more (mean difference = 2.35°, p ≤ 0.090, Cohen’s d = 0.80, 95% CI -0.40, 5.10) of the improvement than the control group (mean change = -6.15° ± 3.44) but this difference was not statistically significant. From baseline measure to 24hr follow-up measure, the TMR group (mean change = 9.90° ± 4.43) displayed significantly improved (mean difference = 10.95°, p ≤ 0.001, Cohen’s d = 2.63, 95% CI 7.79, 14.11) PSLR values compared to the control group (mean change = -1.05° ± 1.72).
The purpose of this study was to determine the effect of a single bout of the TMR® Fab 6 assessment and treatment to increase hamstring flexibility in healthy participants as measured by the AKET and PSLR tests. The results suggest that both the control group and the TMR® group demonstrated immediate post-intervention change in hamstring flexibility, however, the increase experienced by the TMR® group was significantly greater than the change experienced by the control group. Further, only the TMR® group experienced a significant change in flexibility over the 24-hour period, denoting the application of TMR® was more effective at producing longer lasting improvements. The large effect sizes (i.e., Cohen’s d) reported across all measures of AKET and PSLR demonstrates a practical significance denoting increased confidence that the difference between the TMR® and control groups is meaningful to clinicians. The results of this exploratory observational study indicate the use of TMR® is more effective for acutely improving measures of hamstring flexibility and maintaining those changes at a 24-hour follow-up than the control group. The magnitude of difference between the TMR® and control groups suggests that a regionally interdependent approach to the treatment of restricted tissue leads to better patient outcomes.
Among researchers, a consensus has not been established for the most effective and efficient method to increase hamstring flexibility Both PNF and SS have been shown to significantly increase ROM.12,30‑33 but the lasting effect of these techniques remains inconclusive.4,34 Depino et al.,34 utilized a standing static hamstring stretching protocol and evaluated ROM using the AKET and found a significant increase in hamstring extensibility at one- and three-minutes post intervention but returned to baseline
after six minutes. In contrast, DeWeijer et al.4 used static stretching with and without an active warm-up and saw a significant increase in AKET ROM which was maintained for a 24-hour period. Unlike traditional stretching methods which produce a positional sensitivity in the golgi tendon organs by affecting the series elastic component of the muscle,4 TMR® promotes the restoration of symmetry and a reduction in restriction throughout the body, resulting in more efficient movement. The current results benefit from a regional interdependence-informed treatment protocol which may produce changes in the passive mechanical properties of the hamstring without intentionally attempting to lengthen the target tissue. It should be acknowledged that recent literature has questioned the impact of asymmetry on movement dysfunction hypothesizing that asymmetry may not be clinically relevant and is considered a normal part of human structure and function.35,36 Treatment paradigms that target bilateral asymmetries may have little to no effect on sport performance or reduction of injury.35
Findings of the present study support previous investigations using the TMR® treatment protocol for increasing ROM. Gamma et al.19,20 found significant increases in shoulder internal and external ROM in baseball players when comparing the TMR® trunk twist and arm raise to a traditional baseball warm-up. Similarly, Dexter et al.37 observed significant increases in internal and external hip ROM in overhead athletes utilizing the TMR forward flexion trunk twist combined with the seated straight leg raise. Unlike the previous TMR® research, the treatment protocol used in the current study involved the application of TMR® across all three sections of the body (i.e., upper extremity, trunk/core, and lower extremity). The authors conclude that the application of the TMR® protocol may only need to occur at the areas of the body with the greatest movement impairment and dysfunction. Additionally, the current results, when combined with previous TMR® research, indicate that statistically significant increases in ROM are possible without the need for utilizing an intervention (e.g., stretching) that targets “tight” tissue to improve flexibility and ROM. Thus, changes in hip ROM and hamstring extensibility may be attributed to a reduction of restriction throughout the entire body as opposed to being the result of “tight” or “shortened” muscles that need to be stretched. Previous researchers have speculated that the positive effects of TMR® may be attributed to neuro-physiological adaptions associated with the increase motor output from spinal neurons.16,37,38 Mechanisms such as neural coupling39 and cross education40 influence the fascial and muscular tissue via integrated central and peripheral nervous system feedback, promoting accommodations in joint ROM and muscular flexibility.37 Using a treatment paradigm focused on the “good” side has been shown to produce bilateral improvements while avoiding the need to increase tension on a target muscle or reinforce a dysfunctional pattern.41
Limitations are present for generalizing the results outside of the study population. Because the research design included only healthy subjects with a mean age of 17.8±1.2
years of age, the results of the current study may not be observed in individuals with hamstring or lumbar pathology, or those who are outside of this age range. Similarly, there are numerous stretching techniques and we only compared the TMR® application to the control group. Additionally, the authors did not collect more long-term follow-up measurements (e.g., 72 hours) or assess other functional measures (e.g., Functional Movement Screen™, EMG analysis) to determine the long-term results of application or examine potential mechanisms of action for the effect of the TMR® protocol.
Future research should examine the use of TMR® as a soft-tissue treatment protocol and compare it to the use of other common stretching techniques (e.g., SS, PNF stretching) utilized by practicing clinicians to improve hamstring extensibility and ROM. The long-term benefits of TMR®, as well as the effects of TMR® on other soft tissue restrictions throughout the body, should be considered in future research. It may also be beneficial to assess the effects of multiple TMR® treatment applications, as well as collect other variables (e.g., EMG data) to assess the potential mechanism of action and determine overall effectiveness of TMR® as a therapeutic intervention.
The results of the current study demonstrate that a single bout of TMR® using one identified lower extremity, one upper extremity, and a trunk twist movement significantly in-
creased measures of hamstring length in healthy participants. Improvements found immediately post-intervention were maintained for the 24-hour follow-up without any direct treatment to the soft tissue of the hamstring. The results of the study suggest that utilizing a regional interdependence approach and balancing the asymmetries in the musculoskeletal system may result in improvements in hamstring extensibility and hip ROM.
Rich D. Patterson DAT, LAT, ATC Department of Exercise, Health & Sport Sciences Pennsylvania Western University California PA, 15419 Rpatterson@pennwest.edu
The authors report no conflicts of interest.
Submitted: December 08, 2023 CDT, Accepted: May 29, 2024 CDT
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Weppler CH, Magnusson SP Increasing muscle extensibility: A matter of increasing length or modifying sensation? Phys Ther. 2010;90(3):438-449. doi:10.2522/ptj.20090012
2. Marshall PWM, Siegler JC. Lower hamstring extensibility in men compared to women is explained by differences in stretch tolerance. BMC Musculoskelet Disord 2014;15(1):223-230. doi:10.1186/1471-2474-15-223
3. Law RYW, Harvey LA, Nicholas MK, Tonkin L, De Sousa M, Finniss DG. Stretch exercises increase tolerance to stretch in patients with chronic musculoskeletal pain: A randomized controlled trial. Phys Ther 2009;89(10):1016-1026. doi:10.2522/ ptj.20090056
4. de Weijer VC, Gorniak GC, Shamus E. The effect of static stretch and warm-up exercise on hamstring length over the course of 24 hours. J Orthop Sports Phys Ther 2003;33(12):727-733. doi:10.2519/ jospt.2003.33.12.727
5. Bandy WD, Irion JM, Briggler M. The effect of static stretch and dynamic range of motion training on flexibility of the hamstring. J Orthop Sport Phys Ther. 1998;27(4):295-300. doi:10.2519/ jospt.1998.27.4.295
6. Cipriani DL, Abel B, Pirrwitz D A Comparison of two stretching protocols on hip range of motion: Implications for total daily stretch duration. J Strength Cond Res 2003;17(2):274-278. doi:10.1519/ 00124278-200305000-00008
7. Magnusson SP, Simonsen EB, Aagaard P, Kjaer M. Biomechanical responses to repeated stretches in human hamstring muscle in vivo. Am J Sports Med. 1996;24(5):622-628. doi:10.1177/ 036354659602400510
8. Wainner RS, Whitman JM, Cleland JA, Flynn TW Regional interdependence: A musculoskeletal examination model whose time has come. J Orthop Sport Phys Ther. 2007;37(11):658-660. doi:10.2519/ jospt.2007.0110
9. Sueki DG, Chaconas EJ. The effect of thoracic manipulation on shoulder pain: A regional interdependence model. Phys Ther Rev. 2011;16(5):399-408. doi:10.1179/ 1743288X11Y.0000000045
10. Cibulka MT Low back pain and its relation to the hip and foot. J Orthop Sport Phys Ther 1999;29(10):595-601. doi:10.2519/ jospt.1999.29.10.595
11. Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sport Phys Ther 2009;39(1):12-19. doi:10.2519/jospt.2009.2885
12. Feland JB, Myrer JW, Merrill RM. Acute changes in hamstring flexibility: PNF versus static stretch in senior athletes. Phys Ther Sport. 2001;1(4):186-193. doi:10.1054/ptsp.2001.0076
13. Decoster LC, Cleland J, Altieri C, Russell P The effects of hamstring stretching on range of motion: A systematic literature review J Orthop Sports Phys Ther 2005;35(6):377-387 doi:10.2519/ jospt.2005.35.6.377
14. Ghanbari A, Ebrahimian M, Mohamadi M, NajjarHasanpour A. Comparing hold relax-proprioceptive neuromuscular facilitation and static stretching techniques in management of hamstring tightness. Indian J Physiother Occup Ther 2013;33(12):727-733.
15. O’Hora J, Cartwright A, Wade CD, Hough AD, Shum GL. Efficacy of static tretching and proprioceptive neuromuscular facilitation stretch on hamstrings length after a single session. J Strength Cond Res. 2011;25(6):1586-1591. doi:10.1519/ JSC.0b013e3181df7f98
16. Baker RT, Hansberger BL, Warren L, Nasypany A. A novel approach for the reversal of chronic apparent hamstring tightness: A case report. In J Sports Phys Ther. 2015;10(5):723-733.
17. Powers CM, Ward SR, Fredericson M, Guillet M, Shellock FG. Patellofemoral kinematics during weight-bearing and non-weight-bearing knee extension in persons with lateral subluxation of the patella: A preliminary study J Orthop Sport Phys Ther. 2003;33(11):677-685. doi:10.2519/ jospt.2003.33.11.677
18. Dalonzo-Baker T Total Motion Release Seminars. Accessed April 20, 2019. http://www.totalmotionpt.com
19. Gamma SC, Baker RT, Iorio S, Nasypany A, Seegmiller JG. A total motion release warm-up improves dominant arm shoulder internal and external rotation in baseball players. In J Sports Phys Ther 2014;9(4):509-517
20. Gamma SC, Baker R, May J, Seegmiller JG, Nasypany A, Iorio SM. Comparing the immediate effects of a Total Motion Release warm-up and a dynamic warm-up protocol on the dominant shoulder in baseball athletes. J Strength Cond Res 2020;34(5):1362-1368. doi:10.1519/ JSC.0000000000002229
21. Carcia CR, Cacolice PA, McGeary S. Defining lower extremity dominance: The relationship between preferred lower extremity and two functional tasks. In J Sports Phys Ther 2019;14(2):188-191. doi:10.26603/ijspt20190188
22. McGrath TM, Waddington G, Scarvell JM, et al. The effect of limb dominance on lower limb functional performance - A systematic review J Sport Sci. 2016;34(4):289-302. doi:10.1080/ 02640414.2015.1050601
23. Martonick NJP, McGowan GP, Baker RT, Larkins LW, Seegmiller JG, Bailey JP Examining movement asymmetries during three single leg tasks using interlimb and single subject approaches. Phys Ther Sport. 2023;63:24-30. doi:10.1016/j.ptsp.2023.07.001
24. Cacolice PA, Starkey BE, Carcia CR, Higgins PE. Research dominance definitions may not identify higher risk limb for anterior cruciate ligament injury in NCAA D3 student-athletes. In J Sports Phys Ther. 2022;17(4):622-627 doi:10.26603/001c.35593
25. Davis DS, Quinn RO, Whiteman CT, Williams JD, Young CR. Concurrent validity of four clinical tests used to measure hamstring flexibility J Strength Cond Res 2008;22(2):583-588. doi:10.1519/ JSC.0b013e31816359f2
26. Gnat R, Kuszewski M, Koczar R, Dziewonska A. Reliability of the passive knee flexion and extension tests in healthy subjects. J Manipulative Physiol Ther. 2010;33(9):659-665. doi:10.1016/j.jmpt.2010.09.001
27 Reurink G, Goudswaard J, Oomen HG, et al. Reliability of the acture and passive knee extension test in acute hamstring injuries. Am J Sports Med 2013;41(8):1757-1761. doi:10.1177/ 0363546513490650
28. Gabbe BJ, Bennell KL, Wajswelner H, Finch CF. Reliability of common lower extremity musculoskeletal screening tests. Phys Ther Sport. 2004;5(2):90-97 doi:10.1016/S1466-853X(04)00022-7
29. Rosnow RL, Rossenthal R, Rubin DB. Contrasts and correlations in effect-size estimation. Psych Sci. 2000;11(6):446-453. doi:10.1111/1467-9280.00287
30. Lim KI, Hyung-Chun N, Kyoung-Sim J. Effects of hamstring muscle extensibility, muscle activity, and balance of different stretching techniques. J Phys Ther Sci. 2014;26:209-213. doi:10.1589/jpts.26.209
31. Yildirum MS, Ozyurek S, Tosun O, Uzer S, Gelecek N. Comparison of effects of static, proprioceptive neuromuscular facilitation and Mulligan stretching on hip flexion range of motion: A randomized controlled trial. Biol Sport 2016;33(1):89-94. doi:10.5604/20831862.1194126
32. Puentedura EJ, Huijbrigts PA, Celeste S, et al. Immediate effects of quantified hamstring stretching: Hold-relax proprioceptive neuromuscular facilitation versus static stretching. Phys Ther Sport. 2011;12:122-126. doi:10.1016/j.ptsp.2011.02.006
33. Lempke L, Wilkinson R, Murray C, Stanek J. The effectiveness of PNF versus static stretching on increasing hip-flexion range of motion. J Sport Rehabil 2018;27(3):289-294. doi:10.1123/ jsr.2016-0098
34. DePino GM, Webright WG, Arnold BL. Duration of maintained hamstring flexibility after cessation of an acute static stretching protocol. J Athl Train. 2000;35(1):56-59.
35. Alfonso J, Pena J, Sa M, Virgile A, Garcia-deAlcaraz A, Bishop C. Why sports should embrace bilateral asymmetry: A narrative review Symmetry 2022;14(10):1993-2011. doi:10.3390/sym14101993
36. Helme M, Tee J, Emmonds S, Low C. Does lowerlimb asymmetry increase injury risk in sport? A systematic review Phys Ther Sport 2021;49:204-213. doi:10.1016/j.ptsp.2021.03.001
37. Dexter RR, Loftis TK, Pettaway AN, Baker RT, May J. The immediate effects of a Total Motion Release warm-up on active rotational hip range of motion in overhead athletes. J Sports Phys Ther 2019;14(6):898-910. doi:10.26603/ijspt20190898
38. Fimland MS, Helgerud J, Solstad GM, Iversen VM, Leivseth G, Hoff J. Neural adaptations underlying cross-education after unilateral strength training. Eur J Appl Physiol. 2009;107:723-739. doi:10.1007/ s00421-009-1190-7
39. Zehr E, Duysens J. Neural coupling between the arms and leg movement during human locomotion. Neuroscientist. 2004;10(4):347-361. doi:10.1177/ 1073858404264680
40. Huang H, Ferris D Upper and lower limb muscle activation is bidirectional and ipsilaterally coupled. Med Sci Sports Exerc 2009;41(9):1778-1789. doi:10.1249/MSS.0b013e31819f75a7
41. Kofotolis N, Kellis E. Cross-training effets of a proprioceptive neuromuscular facilitation exercise programme on knee musculature. Phys Ther Sport 2007;8(41):109-116. doi:10.1016/j.ptsp.2007.02.004

Rabin A, Noyman L, Yaakobi N, Kazum E. Apprehension-Based Training: A Novel Treatment Concept for Anterior Shoulder Dislocation – A Case Report. IJSPT. Published online July 1, 2024:888-897. doi:10.26603/001c.118928
Alon
Rabin1a , Livneh Noyman1 , Noa Yaakobi1 , Efi Kazum2,3
1 Physical Therapy, Ariel University, 2 Orthopaedic Surgery, Tel Aviv Sourasky Medical Center, 3 Sackler Faculty of Medicine, Tel-Aviv University
Keywords: Rehabilitation, Anterior Shoulder Dislocation, Apprehension https://doi.org/10.26603/001c.118928
Background and Purpose
Conservative management of anterior shoulder dislocation (ASD) is associated with greater recurrence compared with surgical management. Current rehabilitation protocols may not adequately challenge shoulder stability to encourage adaptive coping strategies. Apprehension-based training (ABT) is a new treatment concept derived from the supine moving apprehension test (SMAT), a previously validated performance measure among patients with ASD The purpose of this case report is to describe the application of ABT in a patient with recurrent ASD.
Study Design
Case report
Case Description
The subject was a 23-year-old male with bilateral recurrent ASD The subject underwent a 17-week exercise program involving gradual exposure to increased anterior instability loads based on the SMAT movement pattern. The Western Ontario Shoulder Instability Index (WOSI), Patient-Specific Functional Scale (PFPS), Tampa Scale of Kinesiophobia, SMAT, shoulder internal and external rotation muscle strength were measured via hand-held dynomometry before and after training.
Outcomes
Following treatment, clinically meaningful gains in quality of life (WOSI) and shoulder function (PSFS) were noted. Kinesiophobia decreased, SMAT and shoulder internal rotator strength increased beyond their respective minimal detectable change. Four months after treatment, quality of life and shoulder function remained improved, and the subject reported a reduced rate of ASD.
Discussion
Apprehension-based training involving gradual exposure to shoulder instability loads may hold potential for improving the management of patients with ASD. Further testing of this concept is warranted.
Level of Evidence
4, single case report
a
Corresponding author: Alon Rabin
Ariel University, Department of Physical Therapy
Kiryat Hamada, P. O. Box 3, Ariel, Israel. Cell: 011-972-52-3581550; Work: 011-972-3-9066398 alonrabin@gmail.com
Anterior shoulder dislocation (ASD) is common among young and active individuals.1,2 While surgical management is associated with lower rates of recurrence,3 most people seem to prefer to avoid surgery after a first-time ASD.4 Conservative management is also the initial treatment of choice by many athletes who wish to resume competitive participation within the same sporting season.5,6 This choice is not without consequence as individuals following ASD exhibit fear of re-injury and fear of movement which often lead to avoidance behavior and a resultant lower quality of life.7‑9
Evidence supporting the conservative management of ASD is insufficient. One case series lacks a detailed description of the rehabilitation protocol,10 while the exercise protocol described in another case series does not include any exercise in the apprehension position (i.e. shoulder abduction and external rotation) which, in the opinion of the authors, may not challenge anterior shoulder stability adequately 11 A single randomized controlled trial indicates better functional outcomes after a neuromuscular exercise program compared with home strengthening exercises.12 Adequate exposure to conditions simulating ASD seems particularly important to stimulate the development of effective neuromuscular coping strategies. Exposure to activities considered fearful by patients with ASD may also serve to lessen the fear associated with shoulder movement and facilitate greater participation in daily and recreational activities. Such an approach has been previously shown beneficial among patients with chronic low back pain.13,14
The supine moving apprehension test (SMAT)15 is a physical performance measure designed to assess the ability to control excessive shoulder horizontal abduction and external rotation which are often implicated in the mechanism of ASD.16 The SMAT movement pattern may also serve as a mechanism to train patients with shoulder instability to develop effective neuromuscular control strategies that may allow for improved coping with anterior instability Additionally, a progressive exercise intervention derived from the SMAT may provide graded exposure to conditions that cause fear, which may help decrease apprehension, and promote greater confidence and willingness to use the shoulder Apprehension-based training (ABT) is a new treatment concept derived from the SMAT. The purpose of this case report is to describe the application of ABT in a patient with recurrent ASD
A 23-year-old left-handed male (height: 181 cm, weight: 81 kg) electrical engineering student presented with complaints of a seven-year history of bilateral shoulder instability. The subject reported dislocating his left shoulder for the first time at the age of 17 while playing soccer The shoulder was reduced on-field with the help of a paramedic. One month later the subject suffered a right ASD also while playing soccer, but this time the shoulder was self-reduced
on field. Over the years the subject described multiple bilateral ASD’s, more frequently on the left side than the right. The subject reported experiencing a left ASD at least once monthly and a right ASD once every few months. These dislocations were typically self-reduced. A magnetic resonance arthrography (MRA) of the left shoulder demonstrated an anterior-inferior labral tear as well as a Hill-Sachs lesion. An MRA of the right shoulder demonstrated an anterior inferior and posterior labral tear and a chronic Hill Sachs lesion. Given the subject’s preference for conservative management he was referred multiple times to physical therapy over the years. Informed consent was obtained from the subject prior to collecting history and physical examination.
Observation revealed no atrophy over the rotator cuff or deltoid, scapular alignment was symmetrical with no apparent winging or anterior tilting, no biceps abnormality or acromioclavicular step deformity were noted. Active range of motion (ROM) of the right/left shoulder into forward flexion (163°/169°), abduction (180°/173°), external rotation (with arm held by the side) (60°/55°) and internal rotation in the behind the back position (to the level of T3/T4 spinous process) were within the normal limits for a young male.17,18 Passive flexion ROM could not be tested in the supine position and passive external rotation ROM could not be tested in the supine or standing position due to apprehension. Passive internal rotation at 90° of abduction of the right/left shoulder was within normal limits (73°/68°, respectively).19 Shoulder internal rotator (IR) and external rotator (ER) muscle strength was tested in the supine position using a hand-held-dynamometer (HHD) with the shoulder positioned at 90° of abduction and neutral rotation (the preferred position of 90° abduction and 90° of external rotation could not be assumed due to apprehension). The dynamometer was placed over the dorsal (for ER strength) or volar (for IR strength) aspect of the distal forearm and two, 5-second repetitions of a “make” test were performed with the subject exerting maximal ER or IR effort and the examiner providing an equal and opposing resistance through the HHD The repetition yielding the highest strength value was recorded. Baseline ER/IR strength on the right was 21.1/18.7 kg, corresponding to an ER/IR ratio of 1.12 and on the left was 16.0/20.8 kg (ER/IR ratio 0.77). The ER/IR ratio measured in this testing position in an overhead athletic population is reported to be 0.91 and 0.94 on the dominant and non-dominant shoulder, respectively 20
The Western Ontario Shoulder Instability Index (WOSI) –The WOSI is a 21-item shoulder instability-related quality of life measure. Each item is scored on a 100 mm visual analogue scale resulting in a total score between 0 – 2100 which can be converted to a percentage with greater scores indicating greater disability 21 The WOSI has excellent testretest reliability (intraclass correlation coefficient (ICC)
0.95,21 and a minimal clinically important difference (MCID) of 220 points (10.4%).22
Tampa Scale of Kinesiophobia (TSK) – The TSK is a 17-item fear of movement and reinjury measure. Each item is scored on a 4-point Likert scale for a total score between 17 to 68 with scores greater than 37 representing high fear of movement.23,24 The MDC of the TSK has been reported as 10 points.25
The WOSI and TSK were administered first immediately following informed consent (pre-test) and again 8 days later (baseline) to establish their stability. The WOSI and TSK scored 84.5% and 50 points, respectively during pre-test, and 88.6% and 52 points during baseline.
The Patient-Specific Functional Scale (PSFS) – The PSFS is a self-reported functional ability measure. The subject selects three daily activities affected by their condition and rates the level of difficulty in performing these activities on three separate 11-point (0 – 10) numeric subscales with 0 indicating “ no difficulty” and 10 indicating “complete inability”. The average score of the three subscales serves as the total score of the PSFS. The PSFS has shown high testretest reliability among patients attending physical therapy for shoulder complaints with an ICC 0.87 (95% CI 0.72, 0.94) an MDC 1.0, and an MCID 1.3.26 The subject’s baseline PSFS score was 7.7/10.
Anterior apprehension test – The anterior apprehension test which is typically scored dichotomously (positive/negative) was rated on a 4-point (0 - 3) ordinal scale for the purpose of this study, with a higher score representing less apprehension. Rating was based on the phase of the test in which apprehension was elicited as detected by resistance to further movement, or verbal expression of fear The examiner stood behind the sitting patient and stabilized the scapula to prevent retraction. The examiner then abducted the shoulder to 90° (score 0), externally rotated the shoulder maximally (score 1), horizontally abducted the shoulder behind the frontal plane (score 2), and finally abducted the shoulder beyond 90° (score 3). The test was scored 0/3 bilaterally during baseline assessment.
Supine moving apprehension test - The SMAT is performed in a supine position by repetitively moving the arm to 135° and 180° of shoulder abduction over a 1-minute period while holding a dumbbell equaling 3% body mass.15
The test is scored based on the number of repetitions completed over a minute and has been shown to possess construct and concurrent validity among patients with ASD 15
The ICC and MDC of the SMAT are 0.84 and 10 repetitions and 0.74 and 12 repetitions on the dominant- and nondominant side, respectively 15
For safety reasons the procedure for the SMAT begins with assessment of the ability to control both end-range positions of the test (i.e. 135° and 180° of shoulder abduction in a supine lying position with the elbow straight while holding a 2 kg weight). If the subject is unable to control either position the SMAT is scored 0. If the subject can control both positions the dynamic test is performed beginning with the weight held in front of the chest. The subject then moves the shoulder to 135° of abduction, returns to the starting position and then moves the shoulder to 180° of
abduction and then returns to the starting position. Completion of this cycle consists of one repetition of the test.15 As the subject was unable to place the shoulder in either position (135° or 180°) on either side the SMAT was scored 0 bilaterally during baseline assessment.
The history, physical examination, and imaging findings suggested the subject presented with bilateral, chronic, recurrent ASD Based on the scores of all self-reported measures and the clear apprehension and unwillingness to move during ROM testing it was apparent that the subject presented with a markedly reduced quality of life due to shoulder instability and high levels of fear of movement. The subject was scheduled to begin treatment one week following baseline assessment.
Treatment was provided by a fellowship-trained sports physical therapist with 25 years of experience in managing musculoskeletal shoulder disorders. Two physical therapist students who had completed all didactic education in musculoskeletal physical therapy were responsible for coordinating visits to the clinic, administering self-reported outcome measures, and maintaining weekly phone contact with the subject to ensure compliance with the exercise program.
The ABT was designed to enable the subject to achieve a SMAT score of a healthy young male population (i.e. ≥ 30 repetitions/minute).15 This approach was believed to progressively increase loads on anterior shoulder stability as well as to gradually expose the subject to fearful conditions. The ABT, which is described in detail in Table 1, is comprised of three phases: endurance (4 weeks), dynamic (5 weeks), and neurocognitive (4 weeks). Each phase consists of one exercise which is performed daily and becomes progressively more challenging with each consecutive week by altering joint position, weight, speed of movement, or cognitive load. A clinic-based treatment session was scheduled each week to verify proper performance and determine readiness to progress. Progression was contingent upon completion of the previous week’s exercise with satisfactory demonstration, as well as the subject’s judgment of readiness to progress. The next level of exercise was then demonstrated to the subject and practiced in front of the physical therapist. If both subject and therapist agreed it was possible to progress, the exercise was performed during the following week until the next treatment session.
The aim of the endurance phase was to increase the ability of the glenohumeral adductors, extensors, and internal rotators to control outer range shoulder horizontal abduction and external rotation moments. Concomitantly, this allowed for a gradual exposure to shoulder positions that are typically avoided due to apprehension. The gradation of forces was achieved by progressing from a lighter to a heavier weight and by progressing from training in the scapularto the frontal-plane (Figure 1, a – d).
Table 1. Apprehension-based exercise program description.
The aim of the dynamic phase was to train the subject to produce and control the dynamic movement pattern of the SMAT (repeatedly extending the arm to 135° and 180° of abduction while lying in a supine position and holding a 2 kg weight). Gradation of forces was achieved through a progressive increase of the pace of movement up to 30 repetitions-per-minute (RPM) which is thought to represent normal SMAT performance (Supplementary videos 1 – 3).15
The initial pace (10 RPM) was set based on a shared therapist-patient decision, and an attempt was made to increase the pace by five repetitions each consecutive week. The subject used a metronome application on his smartphone to help maintain the prescribed exercise pace. Increasing movement pace was thought to place greater horizontal abduction and external rotation momentum which was hypothesized to further challenge anterior shoulder stability
• 30 X 10 seconds at 135° abduction
• 30 X 10 seconds at 180° abduction
• 30 X 10 seconds at 135° abduction
• 30 X 10 seconds at 180° abduction
• 30 X 10 seconds at 135° abduction
• 30 X 10 seconds at 180° abduction
• 30 X 10 seconds at 135° abduction
• 30 X 10 seconds at 180° abduction
• 3 X 1 minute at 10 RPMb
• 3 X 1 minute at 15 RPM
• 3 X 1 minute at 20 RPM
• 3 X 1 minute at 25 RPM
• 3 X 1 minute at 30 RPM
• 3 X 1 minute at 25 RPM
• 3 X 1 minute at 30 RPM
• 3 X 1 minute at 25 RPM
• 3 X 1 minute at 30 RPM
Random order of movement. One arm at a time
Random order of movement. Both arms simultaneously
and concomitantly expose the subject to more fearful conditions.
During the neurocognitive phase the subject continued to perform the SMAT movement pattern at a relatively high pace (25 – 30 RPM) however the order of arm movement (135° or 180°) was unpredictable and in response to numeral commands. Audio files including randomly ordered double-digit numbers comprised of the numerals 1 and 2, or 3 and 4 (i.e., 11, 21, 34, 44) were pre-recorded and sent to the subject’s smartphone. The subject was instructed to extend the left arm to the 135° position in response to the numeral “1”, extend the left arm to the 180° position in response to the numeral “2”, extend the right arm to the 135° position in response to the numeral “3”, and extend the right arm to the 180° position in response to the numeral “4”. Accordingly, in response to the number “11” the
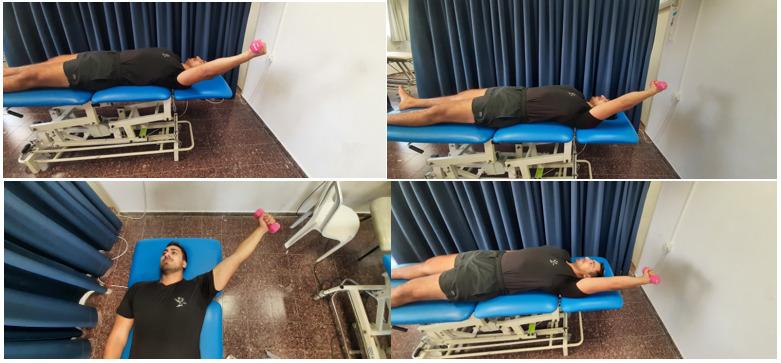
Figure 1. Endurance phase: (a) 135° scapular-plane abduction; (b) 180° scapular-plane abduction; (c) 135° frontal-plane abduction; (d) 180° frontal-plane abduction.
subject was required to extend the left arm to the 135° position twice in a row, and in response to the number “43” the subject was required to extend the right arm to the 180° position followed by the 135° position. During the first two weeks of the neurocognitive phase the subject exercised each arm separately while during the last two weeks both arms were trained simultaneously (Supplementary videos 4, 5). The neurocognitive phase incorporates elements of attention, dual-tasking, reaction time, and memory. The addition of cognitive demands to a physical task has been previously shown to decrease performance during lower extremity functional tests such as hopping and a change of direction.27,28 The inclusion of such elements is in accordance with current views on the need to design rehabilitation procedures and return-to-play testing to simulate the chaotic conditions of competitive sports more closely.29 Following the completion of the intervention the subject was scheduled for a final assessment which included the three self-reported outcome measures (WOSI, TSK, PSFS), a repeat examination of IR and ER muscle strength, the anterior apprehension test and SMAT Four months after treatment the subject was contacted to report any additional instability episodes and to again complete the WOSI, TSK and PSFS.
The subject was able to complete the intervention within 17 weeks. The program lasted four weeks longer than expected as during the 3rd week of the program (endurance phase) the subject suffered a left shoulder dislocation while reaching into the backseat of his car. The dislocation was selfreduced within a few seconds, however this resulted in the need to rest the shoulder for 10 days. In addition, the subject needed an extra two weeks of training to progress from a pace of 25 to 30 RPM during the dynamic phase (week 8),
as well as an extra week of training to progress from a pace of 25 to 30 RPM during the neurocognitive phase (week 12).
At the end of the training period the subject reported greater confidence and willingness to use the shoulder during daily activities. Table 2 summarizes pre-test, baseline, post-treatment, and follow-up self-reported measures, while Table 3 summarizes baseline and post-treatment physical examination measures.
The WOSI and PSFS improved well beyond their respective MCID following treatment,22,26 while the TSK score improved beyond its reported MDC.25 The SMAT score of both shoulders increased well beyond their respective MDC,15 and shoulder IR muscle strength improved beyond the MDC previously reported for a similar testing methodology 20 Shoulder ER muscle strength remained unchanged. The anterior apprehension test was still positive bilaterally, but apprehension was elicited in a more advanced stage of the test in both shoulders. Finally, passive ROM into shoulder flexion and external rotation was possible with no apprehension and measured 175°/170° and 80°/86° on the right/left side.
Four months after treatment the WOSI and PSFS remained improved, however TSK score increased close to its baseline level (Table 2). The subject reported one additional dislocation of the right shoulder which occurred immediately after the treatment period as he was getting dressed after an MRA of his right shoulder. The dislocation was self-reduced, and no additional dislocations have occurred since. Therefore, over an 8-month period (from the beginning of treatment to four months following treatment) the subject experienced one dislocation of each shoulder The subject considered this to be a clear reduction of the rate of shoulder dislocations. Finally, the subject reported continuing to exercise at a frequency of once a week.
Table 2. Pre-test, baseline, final, and follow-up self-reported outcome measures.
PSFS, Patient-specific Functional Scale; TSK, Tampa Scale of Kinesiophobia; WOSI, Western Ontario Shoulder Instability Index. a Minimal
Table 3. Pre- and post-treatment physical examination measures.
Left anterior apprehension (0 – 3)
ER, external rotation; IR, internal rotation; SMAT, supine moving apprehension test.
A novel exercise program specifically designed for patients with ASD was successfully implemented in the care of a patient with bilateral recurrent ASD Following intervention clinically meaningful gains in quality of life and functional ability were observed along with reduced kinesiophobia and increased willingness to move the shoulder The SMAT score improved beyond the random error associated with this test.15 The change in SMAT score and final SMAT score were well above those observed among patients after surgical stabilization and post-operative rehabilitation.15 Furthermore, the final SMAT score for both shoulders was only slightly below the score of a healthy young male population.15 Collectively, these results suggest ABT may hold potential in improving the conservative care of patients with ASD
The ABT is unique in that it is focused on obtaining control of a specific movement pattern that is known to be deficient among patients with ASD.15 The basic premise behind the program is that confrontation with progressively increased shoulder apprehension conditions will facilitate neuromuscular and psychological adaptations that will result in increased confidence and willingness to use the shoulder during activities of daily living.
Previous studies suggest delayed and reduced activation of muscles with potential to control excessive shoulder horizontal abduction and external rotation such as the pectoralis major and subscapularis.30,31 Such neuromuscular
deficits may play a role in the mechanism of recurrent ADL. The ABT was designed to improve the function of these muscles as well as other muscles with potential to prevent excessive horizontal abduction and external rotation such as the teres major, latissimus dorsi, and biceps brachii. Although the specific muscle activation pattern associated with ABT has not yet been reported, it is theorized that the supine lying position with the shoulder at 135° or 180° of abduction as performed in the endurance phase elicits activation of these muscles. The rapid acceleration of the shoulder into the 135° and 180° positions during the dynamic and neurocognitive phases is thought to invoke reactive activation of the adductors and internal rotators of the shoulder to decelerate the arm at end-range and accelerate it back to the starting position. Gradually increasing the pace of this movement creates plyometric training conditions, which has been previously shown to elicit joint protective neuromuscular adaptations such as improved proprioception and kinesthesia, faster production of maximal torque, and decreased amortization time from external to internal rotation.32 Although the authors did not assess for these adaptations after treatment, there was an increase in shoulder IR but not ER muscle strength following treatment, suggesting muscle-specific adaptations had occurred.
Patients with recurrent ASD have been shown to exhibit increased kinesiophobia,7,8 and persistent avoidance of certain daily and sport-related activities.9 Increased activation of brain networks regulating motor resistance, cognitive
control of movement, anxiety, and emotional regulation has been elicited in patients with anterior shoulder instability when visually presented with movements associated with anterior shoulder apprehension.33 Given these findings, it would seem that patients with ASD may benefit from psychological-based interventions such as graded exposure which has been successfully used among patients with chronic low back pain.14 The ABT provides a simple and feasible platform for providing such graded exposure in the context of ASD The progressive confrontation with anterior instability loads may lead to habituation and degradation of the apprehension response. This may have been manifested by a decrease in kinesiophobia at the end of the treatment period, an increased tolerance to the anterior apprehension test and passive ROM testing, and an improved performance of the SMAT
Despite the decreased rate of ASD and the clinically meaningful improvement in most outcome measures, the subject remained affected by shoulder instability as manifested by a relatively high WOSI score, a positive apprehension test and the recurrence of kinesiophobia at followup. While long-term disability and persistent apprehension have been documented years after surgical stabilization of the shoulder,9,34 incorporating additional elements into ABT should be considered. Exercises in this case report were performed solely in the supine lying position. While this provides for a controlled progression of anterior destabilizing forces, it does not consider the variety of circumstances under which ASD might occur.35 Incorporating the control of the same movement pattern in other positions or under weight-bearing and/or closed kinematic conditions may serve to lessen kinesiophobia and disability even further
Another consideration is adaptability. The ABT may need to be modified based on the individual needs of patients. For example, early after an acute ASD, patients must first regain full active ROM prior to beginning the ABT protocol. In contrast, patients who can perform the SMAT relatively well at baseline may be allowed to progress more rapidly through the initial phase of the program. Finally,
patients presenting with impairments possibly contributing to shoulder instability such as scapular dyskinesis or rotator cuff strength deficits, should be prescribed additional exercises to address such impairments.
This case report has several other limitations. Most importantly, a case report cannot infer a cause-and-effect relationship between intervention and outcome. Only a randomized controlled design can prove whether ABT yields results that differ from those of other interventions or the natural history of ASD Second, no long-term follow was performed, and outcome is limited to the immediate- and short-term periods only Third, the mechanism behind the clinical improvement observed following treatment is unclear Although a decrease in kinesiophobia was evident, possible other mechanisms relating to neuromuscular control were not assessed. Fourth, because no interim outcome assessment was carried out, it is unclear what was the value of each of the three phases of the ABT in obtaining the outcome. And finally, the reliability of the anterior apprehension test as performed and scored in this study was not previously determined and findings relating to this outcome need to be interpreted with caution.
Apprehension-based training, an intervention specifically designed for patients with ASD, was successfully completed by a subject with bilateral recurrent ASD Clinically meaningful gains in quality of life and functional ability were detected, along with a reduced rate of shoulder dislocations, decreased kinesiophobia, increased willingness to move, and an improved SMAT score. Further study is warranted to explore the feasibility and efficacy of this intervention among patients with ASD.
Submitted: November 26, 2023 CDT, Accepted: May 29, 2024 CDT
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Liavaag S, Svenningsen S, Reikeras O, et al. The epidemiology of shoulder dislocations in Oslo. Scan J Med Sci Sports. 2011;21(6):e334-e340. doi:10.1111/ j.1600-0838.2011.01300.x
2. Zacchilli A, Owens BD Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Joint Surg Am 2010;92(3):542-549. doi:10.2106/JBJS.I.00450
3. Belk JW, Wharton BR, McCarty EC, et al. Shoulder stabilization versus immobilization for first-time anterior shoulder dislocation: A systematic review and meta-analysis of Level 1 randomized controlled trials. Am J Sports Med 2022;51(6):1634-1643. doi:10.1177/03635465211065403
4. Yaari L, Ribenzaft SZ, Kittani M, Yassin M, Haviv B. Epidemiology of primary shoulder dislocations requiring surgery: a cohort study from a major trauma center during 7 years. J Orthop Surg (Hong Kong) 2022;30(3):10225536221134032. doi:10.1177/ 10225536221134032
5. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42(12):2842-2850. doi:10.1177/ 0363546514553181
6. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability: Surprising findings in nonoperative management in a high school athlete population. Am J Sports Med. 2019;47(5):1062-1067 doi:10.1177/ 0363546519829765
7. Olds M, Ellis R, Parmar P, Kersten P. The immediate and subsequent impact of first-time traumatic anterior shoulder dislocation in people aged 16-40: Results from a national cohort study. Shoulder Elbow. 2021;13(2):223-232. doi:10.1177/1758573220921484
8. Eshoj H, Rasmussen S, Frich LH, Jensen SL, Sogaard K, Juul-Kristensen B. Patients with nonoperated traumatic primary or recurrent anterior shoulder dislocation have equally poor self-reported and measured shoulder function: A cross-sectional study BMC Musculoskelet Disord 2019;20(1):59. doi:10.1186/s12891-019-2444-0
9. Safran O, Beyth S, Milgrom C, Milgrom Y, Nir D, Finestone AS. At long-term follow-up many first-time male traumatic shoulder dislocators remain symptomatic. J Sci Med Sport. 2023;26(6):291-295. doi:10.1016/j.jsams.2023.03.008
10. Riccio I, de Sire A, Latte C, Pascarella F, Gimigliano F. Conservative treatment of traumatic shoulder instability: A case series study. Musculoskelet Surg 2015;99:133-137 doi:10.1007/ s12306-015-0373-0
11. Aronen JG, Regan K. Decreasing the incidence of recurrence of first-time anterior shoulder dislocations with rehabilitation. Am J Sports Med 1984;12:283-291. doi:10.1177/036354658401200408
12. Eshoj HR, Rasmussen S, Frich LH, et al. Neuromuscular exercises improve shoulder function more than standard care exercises in patients with traumatic anterior shoulder dislocation: A randomized controlled trial. Orthop J Sports Med 2020;8(1):2325967119896102. doi:10.1177/ 2325967119896102
13. George SZ, Zeppieri G. Physical therapy utilization of graded exposure. J Orthop Sports Phys Ther 2009;39(7):496-505.
14. Woods MP, Asmundson GJG. Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. Pain 2008;136(3):271-280. doi:10.1016/j.pain.2007.06.037
15. Rabin A, Chechik O, Olds MK, et al. The supine moving apprehension test – reliability and validity among healthy individuals and patients with anterior shoulder instability. Shoulder Elbow. Published online 2023. doi:10.1177/17585732231170197
16. McMahon PJ, Chow S, Sciaroni L, Yang BY, Lee TQ. A novel cadaveric model for anterior-inferior shoulder dislocation using forcible apprehension positioning. J Rehabil Res Dev. 2003;40:349-359. doi:10.1682/JRRD.2003.07.0349
17 Gill TK, Shanahan EM, Tucker GR, Buchbinder R, Hill CL. Shoulder range of movement in the general population: Age and gender stratified normative data using a community-based cohort. BMC Musculoskelet Disord. 2020;21(1):676. doi:10.1186/ s12891-020-03665-9
18. Constant CR, Gerber C, Emery RJH, Sojbjerg JO, Gohlke F, Boileau P A review of the Constant score: modifications and guidelines for its use. J Shoulder Elbow Surg 2008;17(2):355-361. doi:10.1016/ j.jse.2007.06.022
19. Lunden JB, Muffenbier M, Giveans MR, Cieminski CJ. Reliability of shoulder internal rotation passive range of motion measurements in the supine versus sidelying position. J Orthop Sports Phys Ther. 2010;40(9):589-594. doi:10.2519/jospt.2010.3197
20. Cools AM, Vanderstukken F, Vereecken F, et al. Eccentric and isometric shoulder rotator cuff strength testing using a hand-held dynamometer: Reference values for overhead athletes. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3838-3847. doi:10.1007/s00167-015-3755-9
21. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772. doi:10.1177/03635465980260060501
22. Kirkley A, Griffin S, Dainty K. Scoring systems for the functional assessment of the shoulder Arthroscopy. 2003;19(10):1109-1120. doi:10.1016/ j.arthro.2003.10.030
23. Kori SH, Miller RP, Todd DD Kinesiophobia: A new view of chronic pain behavior. Pain Manag. Published online 1990:35-43.
24. Vlayen JWS, Kole-Snijders AMJ, Boeren RGB, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain 1995;62(3):363-372. doi:10.1016/ 0304-3959(94)00279-N
25. Yona T, Yaniv M, Rom J, Damri E, Fischer AG. Translation, cross-cultural adaptation and reliability of the International Knee Documentation Committee (IKDC) subjective knee form and the tampa scale for kinesiophobia (TSK) into Hebrew Arch Orthop Trauma Surg. 2023;143(5):2629-2640. doi:10.1007/ s00402-022-04548-5
26. Koehorst MLS, Van Trijffel E, Lindeboom R. Evaluative measurement properties of the subjectSpecific Functional Scale for primary shoulder complaints in physical therapy practice. J Orthop Sports Phys Ther. 2014;44(8):595-603. doi:10.2519/ jospt.2014.5133
27 Simon JE, Millikan N, Yom J, Grooms DR. Neurocognitive challenged hops reduced functional performance relative to traditional hop testing. Phys Ther Sports. 2020;41:97-102. doi:10.1016/ j.ptsp.2019.12.002
28. Serpell BG, Ford M, Young WB. The development of a new test of agility for rugby league. J Strength Cond Res. 2010;24(12):3270-3277. doi:10.1519/ JSC.0b013e3181b60430
29. Wilk K, Thomas ZM, Arrigo CA, Davies GJ. The need to change return to play testing in athletes following ACL injury: A theoretical model. Int J Sports Phys Ther 2023;18(1):272-281. doi:10.26603/ 001c.67988
30. Myers JB, Ju YY, Hwang JH, McMahon PJ, Rodosky MW, Lephart SM. Reflexive muscle activation alterations in shoulders with anterior glenohumeral instability Am J Sports Med 2004;32:1013-1021. doi:10.1177/0363546503262190
31. Brostrom LA, Kronberg M, Nemeth G. Muscle activity during shoulder dislocation. Acta Orthop Scand 1989;60:639-641. doi:10.3109/ 17453678909149593
32. Swanik KA, Lephart SM, Swanik CB, Lephart SP, Stone DA, Fu FH. The effects of shoulder plyometric training on proprioception and selected muscle performance characteristics. J Shoulder Elbow Surg 2002;11(6):579-586. doi:10.1067/mse.2002.127303
33. Haller S, Cunningham G, Laedermann A, et al. Shoulder apprehension impacts large-scale functional brain networks. AJNR Am Neuroradiol 2014;35(4):691-697.
34. Maman E, Dolkart O, Krespi R, et al. A multicenter retrospective study with a minimum 5-year follow-up comparing arthroscopic Bankart repair and Latarjet procedure. Orthop J Sports Med 2020;8(8):2325967120941366. doi:10.1177/ 2325967120941366
35. Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy Am J Sports Med 2007;35(7):1168-1173. doi:10.1177/ 0363546506295179
Download: https://ijspt.scholasticahq.com/article/118928-apprehension-based-training-a-novel-treatment-conceptfor-anterior-shoulder-dislocation-a-case-report/attachment/230899.mp4?auth_token=XLCm2sjLQhWAg9bXSNLo
Download: https://ijspt.scholasticahq.com/article/118928-apprehension-based-training-a-novel-treatment-conceptfor-anterior-shoulder-dislocation-a-case-report/attachment/230902.mp4?auth_token=XLCm2sjLQhWAg9bXSNLo
Download: https://ijspt.scholasticahq.com/article/118928-apprehension-based-training-a-novel-treatment-conceptfor-anterior-shoulder-dislocation-a-case-report/attachment/230901.mp4?auth_token=XLCm2sjLQhWAg9bXSNLo
Download: https://ijspt.scholasticahq.com/article/118928-apprehension-based-training-a-novel-treatment-conceptfor-anterior-shoulder-dislocation-a-case-report/attachment/230898.mp4?auth_token=XLCm2sjLQhWAg9bXSNLo
Download: https://ijspt.scholasticahq.com/article/118928-apprehension-based-training-a-novel-treatment-conceptfor-anterior-shoulder-dislocation-a-case-report/attachment/230900.mp4?auth_token=XLCm2sjLQhWAg9bXSNLo

Zeppieri G, Smith MS, Roach RP. Nonsurgical Management of Adductor-related groin pain with Ultrasound-Guided Platelet-Rich Plasma Injection and Physical Therapy in a Competitive Soccer Player: A Case Report. IJSPT. Published online July 1, 2024:898-909. doi:10.26603/001c.120209
Giorgio
Zeppieri1,2a , Micheal S Smith, Ryan
P. Roach
3
1 Rehabilitation, University of Florida, 2 Team Physical Therapist, University of Florida, 3 Department of Orthopaedic Surgery and Sports Medicine , University of Florida
Keywords: Platelet- Rich Plasma Injection, Groin Pain, soccer, adductor-related groin pain https://doi.org/10.26603/001c.120209
Introduction
Adductor-related groin pain involves an injury to the common aponeurosis connecting the rectus abdominus and adductor longus to the pubis. It commonly occurs in sports that require cutting and pivoting and can result in significant loss of playing time.
Platelet-Rich Plasma (PRP) is often indicated for treatment of musculoskeletal disorders and may represent an alternative treatment for patients with adductor-related groin pain. The purpose of this case report is to describe the non-surgical management of adductor-related groin pain in a competitive soccer player with a with an ultrasound (US)-guided PRP injection and physical therapy management.
A 17-year-old male competitive soccer player with right-sided adductor-related groin pain was treated with an US-guided PRP and a multi-phased physical therapy regimen based on tissue healing and individual patient/criteria progression. The patient completed 12 physical therapy sessions over six weeks post PRP injection.
At the end of treatment, clinically meaningful improvements were observed in pain intensity, passive range of motion, strength (handheld dynamometry, Biodex), functional tests, psychosocial (OSPRO-YF) and patient-reported outcomes (HAGOS, LEFS). The subject returned to sport at six weeks post injection without limitation and at three months follow up, the subject reported that he had returned to 95% of his previous level of play
This case report may offer support for PRP as an alternative treatment in the management of adductor-related groin pain. Incorporation of PRP as an adjunct to physical therapy led to improvements on all outcomes that surpassed the clinical significance change criteria.
Level of evidence
5
a
Corresponding Author: Giorgio Zeppieri Jr., PT, SCS Department of Rehabilitation, University of Florida, Team Physical Therapist, University of Florida Health 3450 Hull Rd, Gainesville, FL 32607 (USA) E-mail: zeppig@shands.ufl.edu
Groin pain is responsible for 8 to 18% of injuries in competitive soccer, with an incidence of 0.8 to 1.3 groin-related injuries per 1,000 hours of athletic exposure.1‑3 Adductorrelated groin pain is most prevalent in sports requiring pivoting, cutting, kicking, and change of direction.4 The patho-anatomy involves weakening of or chronic tensile overload at the common attachment of the distal rectus abdominus and proximal adductor longus tendon.5‑8 Symptoms are typically unilateral and exacerbated by sudden sport specific movements.5,9‑11 Pain is often localized to the medial groin and may radiate to the perineum, adductors, distal rectus insertion, inguinal ligament, and/or testicular area.5,6
Treatment options include both nonoperative and operative interventions. Depending on the timing of competitive play (whether preseason or in season), initial treatment focuses on rest, anti-inflammatory medication, and/ or physical therapy.6,8,12 However, nonoperative management has been poorly defined, with outcomes following non-surgical management inconsistently reported.4,12 Furthermore, results after use of corticosteroid injections are inconclusive and have been shown to only provide temporary relief that eventually leading to a surgical intervention.12,13 Surgical intervention has been shown to be more effective than nonsurgical options, however, surgical intervention has risks and access to surgeons that treat adductor-related groin pain may be limited.14 Additionally, surgical intervention may lead to a substantial amount of time loss from competitive play or be season ending.4,12
Platelet-Rich plasma (PRP) has been advocated for use in augmenting the healing progression in chronic injuries and acceleration of acute tissue repair by releasing biologically active elements to help in revascularization and regeneration of connective tissue.15 Further, there is evidence to support the use of PRP in tendinopathic pathologies.8,16‑20 Consequently, PRP may represent a novel modification to the rehabilitation of individuals with adductor-related groin pain with the potential of avoiding surgery, improving outcomes while decreasing time loss from sport. This case describes the non-surgical management of adductorrelated groin pain in a competitive soccer player with an US-guided PRP injection and accelerated physical therapy management.
A 17-year-old competitive soccer player presented to a sports medicine clinic with right-sided chronic groin pain of six months in duration. The injury occurred as he planted with his left leg and struck the ball with his right leg. He noted immediate sharp, stabbing pain along his pubic bone. He reported that his shot was rushed by the defender and as he planted with his left leg and he “ over kicked” with his right leg. He initially rested from athletic activities, but symptoms returned upon resumption of sprinting and soccer-specific activities.
During the initial examination, he reported 0/10 pain level at rest, and 9/10 pain level with activity. His examination revealed localized tenderness and pain in the inguinal canal along the right adductor longus origin and lateral to the umbilicus and distally to the pubis consistent to the insertion site of rectus abdominis. Resisted adductor and partial sit-up tests were provocative e for pain and symptom exacerbation. Pain decreased with rest, modification of soccer specific activities, and ice. His goal was to decrease pain associated with soccer related activities and to return fully to his previous competitive level activity.
PATIENT REPORTED OUTCOMES
To assess hip and groin symptoms, activity limitations, participation restrictions in daily living and sport, and quality of life, the Copenhagen Hip and Groin Outcome Score was used. The HAGOS has been validated in young to middle aged, physically active individuals with hip and groin pain.21 The HAGOS consists of 6 subscales, 7-item symptom assessment scale, 5-item limitation in activity and daily living scale, 8-item limitation in sport participation scale, 2-item participation in physical activity scale, and 5-item quality of lift scale. Each subscale is scored on 5-point Likert scale. Scores in each subscale are summed and divided between maximum score achievable in each subscale and then multiplied by 100. Higher score indicates decreased groin and hip dysfunction. The ICC for the individual HAGOS subscales is between 0.82 to 0.92.21 MCIDs for the HAGOS subscales are symptom assessment (≥10), restrictions in activities of daily living (≥11.2), pain (≥ 9.8), physical activity (≥16.9), quality of life (≥12.7), restriction sport participation (≥13.1).22‑24
The Lower Extremity Functional Scale was used to assess the patient’s lower extremity orthopedic function. The LEFS consists of 20 items, scored from 0 (extremely difficult or unable to do) to 4 (no difficulty). The score is summed and higher scores indicate increased lower extremity function, while lower scores indicate decreased lower extremity function. The ICC for the LEFS is 0.94 in individuals with lower extremity, musculoskeletal dysfunction.25 MCIDs for the LEFS is 9 points.25
The Patient Specific Functional Scale was utilized to assess the patient’s overall function by identifying three patient specific important activities presentably limited by their groin pain. The patient was then asked to score each activity from 0 (unable to perform the activity) to 10 (fully able to perform the activity with restriction) based on their preinjury level. The PSFS is a valid and reliable instrument (ICCs = 0.71-0.85) with a MCID of 1.2 points.26
The OSPRO Yellow Flag Assessment Tool is a 17-item questionnaire that includes items from pain susceptibility (negative coping and fear-avoidance) and confrontation (positive coping and self-efficacy) domains.27 The OSPRO-YF was used to screen for pain-related psychosocial distress
with higher scores indicating higher levels of psychosocial distress.27,28 The OSPRO has been shown good concurrent validity with pain intensity and functional disability across anatomical regions.28
Strength was assessed through maximum voluntary isometric contractions using a handheld dynamometer (MicroFet 2, Hoggan Scientific LLC, Salt Lake City, UT, USA) for gluteus medius, adductor longus, and psoas and isokinetic testing (Biodex Medical System 2.0, Shirley, NY, USA) for quadriceps and hamstring. All dynamometer measurements were recorded in Newtons and included three trial for each measurement, with best of three recorded. The procedure for all testing positions, was to test the uninvolved hip first, followed by the involved hip. The minimal detectable change for hip abduction is 35.5 N (ICC = .85), hip adduction is 30.8 N (ICC = .94), and hip flexion is 80.1 N (ICC= .76).29 All isokinetic measurements (ICC = .82-.95) were performed at 60 deg/sec and recorded for peak torque (ft/lbs).30
Hip abductor strength was measured in lateral decubitus. The hip to be tested was placed on top and placed in slight extension with the knee fully extended, parallel to the table.31,32 The contralateral hip was positioned in 40° of flexion with 90° flexion at the knee.31,32 One examiner stabilized the pelvis with hands placed at the lumbar and anterior iliac while a second examiner positioned the dynamometer force pad proximal to the lateral femoral condyle.31,32 The subject performed a maximal muscle contraction against the dynamometer force pad for five seconds to record a result.
Hip adductor strength was measured supine. The hip to be tested was placed in a neutral position. The contralateral leg was flexed with the patient’s foot fixed on the table. One examiner stabilized the pelvis with their hands along anterior iliac spines, the second examiner positioned the dynamometer force pad superior to the medial malleoulus.31 The patient performed a maximal muscle contraction against the dynamometer force pad for five seconds to record a result.
Hip flexor strength was measured sitting. Both the testing hip and the contralateral hip were positioned in 90 degrees of flexion.31 One examiner stabilized the contralateral leg. The second examiner positioned the dynamometer proximal to the patella.31 The patient performed a maximal muscle contraction against the dynamometer force pad for five seconds to record a result.
Passive range of motion for hip flexion and abduction was measured with a 2 -arm goniometer, while passive hip internal and external rotation was measured with a bubble goniometer All ROM measurements were recorded utilizing a two-tester method and for all testing positions, the uninvolved hip was tested first, followed by the involved hip. The MDC for hip flexion is 8.2° (ICC = 0.97), hip abduction is 7.3° (ICC = .94), hip external rotation is 7.1° (ICC = .98) and hip internal rotation is 7.8° (ICC= .98).33‑35
Hip flexion ROM was measured supine with both legs extended on the table. The first examiner passively flexed the
hip and knee of the testing leg to end ROM. The second examiner aligned a goniometer with the midline trunk and lateral femoral condyle. This method has a reported excellent interclass correlation coefficient (ICC) of 0.97.35,36
Hip abduction ROM was measured supine. The contralateral hip was positioned in slight abduction and the pelvis was stabilized by the first examiner 33 The second examiner placed the center of the goniometer on the testing hip anterior superior iliac spine (ASIS) and positioned the stationary arm of the goniometer towards the contralateral ASIS.33 The moving arm of the goniometer was aligned along the midline of the testing femur 33 The second examiner passively abducted the hip.
Hip external and internal rotation was measured prone. The test leg was placed in 0 degrees of hip extension and abduction with the knee flexed to 90 degrees.34,36‑38 Examiner one stabilized the pelvis by placing their hands over the ischium, while passively moving the lower leg until first resistance was detected.34,36‑38 The second examiner aligned a bubble inclinometer proximal to the medial malleolus in line with the shaft of the tibia.34,36‑38Performance based measures to assess power were measured through series hop testing (single leg, triple, and cross over hop). Measurements were recorded as limb symmetry index (LSI) and included three practice attempts prior to testing. The average of the three trials were calculated and LSI was recorded as the ratio of distance hopped on the injured side as a percentage of the distance hopped on the non-injured side. Reliability index for limb symmetry is (ICC = .92) for the single leg hop, (ICC = .88) for the triple hip, and (ICC = .84) for the cross over hop.39
The subject was screened for associated intra and extra articular hip pathology, as well as lumbar pathology prior to determining adductor-related groin pain as the cause of symptoms. There was no tenderness along the lumbar spine with full, pain free lumbar range of motion. There was intact light touch sensation along dermatomal patterns with normal low extremity reflexes. Straight leg raise and slump test were negative bilaterally. Hip flexion, abduction, and internal rotation (FADIR) as well hip flexion, adduction, and internal rotation (FABER) were assessed to rule out femoroacetabular impingement (FAI) pathology Both tests reproduced his groin pain, however symptoms were mild. Resisted partial sit-up and leg adduction tests did reproduce symptoms.
Passive range of motion (PROM) of hip was limited to 115 degrees of flexion with 5/10 pain with associated muscle guarding, 30 degrees of abduction with 4/10 pain with associated muscle guarding, 31 degrees of internal rotation with 5/10 pain, and 30 degrees of external rotation with 2/ 10 pain. The patient presented with pain and weakness during strength testing using a hand-held dynamometer Table 1 summarizes these findings. The patient had positive findings during the FABER test for reproduction of groin pain, but negative findings for sacroiliac, lumbar, or posterior hip pain. FADIR test was positive for reproduction of groin pain.

Figure 1. Pre-operative coronal short tau inversion recovery (STIR) magnetic resonance image reveals in (A) marrow edema within the right parasymphyseal pubis (solid arrow) and a central fluid cleft along the articular disc of the pubic symphysis (open arrow), and in (B) a left anterosuperior acetabulum labral tear. Sagittal proton density fat-suppressed sequence images demonstrate disc protrusions at L4/5 and L5/S1 (C) and increased signal along the insertion of the left rectus abdominis (D) when compared to the right (E).
Due to the intimate association between FAI, acetabular labral tears, and adductor-related groin pain, a magnetic resonance imaging (MRI) was ordered.12,40,41 There was no evidence on MRI of an acetabular labral tear or cartilage defect. There was stripping of the common adductor/rectus abdominis aponeurosis from the anterior pubic body, right greater than left with mild articular cortical irregularity at the pubic symphysis (Figure 1). There were no findings to suggest iliopsoas or trochanteric bursitis. The surrounding muscles and tendinous attachments were normal. There was no soft tissue mass or muscular atrophy and the visualized intraperitoneal structures and somatic soft tissues of the pelvic sidewalls were also deemed normal. Based on the MRI findings, patient’s subjective complaints and mechanism of injury, and clinical objective findings, adductor-related groin pain was diagnosed. Given the timing of the injury (start of the high school season), commitment to overseas competitive academy post high school season (two months), and reluctance of the athlete to undergo surgical intervention due to anxiety of missing entire of high school season plus delay of overseas professional career, the athlete opted for an Ultrasound (US) guided PRP injection.
Treatment consisted of an US-guided PRP Injection to the right adductor (MSS) followed by a multi-phased physical
therapy program based on tissue healing and individual patient progression twice a week for six weeks.
ULTRASOUND-GUIDED PLATELET-RICH PLASMA INJECTION
The Arthrex Angel System (Naples, FL) was utilized to deliver platelet-rich plasma. The max centrifuge spin was 4000 RPMs with a starting volume of 60 ml of the patient’s own blood and a hematocrit setting of 4%. There was an average fold yield increase of a 5.76 platelet (PLT), 1.69 white blood cell (WBC), and .67 neutrophil (NE) obtained in PRP preparation relative to whole blood. Cellular concentrate included a PLT concentration of 1032.94 k/µL, WBC concentration of 8.55 k/µL, and a NE concentration of 1.81 k/µL. The adductor was identified with ultrasound, and a doppler was used to evaluate for any adjacent neurovascular structures. The injection site was cleansed with chlorhexidine, and sterile gel was applied. 5 ml of 1% lidocaine and 1 mL (40 mg/mL) of Depo Medrol were delivered through the 22-gauge 3 ½ inch needle into the adductor tendon. Subsequently, 5 ml of PRP was delivered through a 20-gauge needle. (Figure 2A &B) Risks, including infection, bleeding, nerve damage, and pain at the injection site were thoroughly discussed with the patient.
Rehabilitation consisted of five phases: immediate post-injection (Phase 1- 1-7 days), controlled motion (Phase 2 –

Figure 2. Ultrasound-guided platelet-rich plasma (PRP) (A) Thickened and disrupted adductor tendon attachment to pubic tubercle (B) Initial trajectory of needle tip and injection of PRP
8-14 days), advanced strengthening (Phase 3- 15-21 days), multi-planar strengthening (Phase 4 – 22-28 days) and return to sport (Phase 5- 28-35 days). During the rehabilitation process, the patient attended scheduled orthopedic medical appointments for routine updates regarding the injection site and rehabilitation progressions. The details of the phased rehabilitation program are provided in Appendix 1.
Phase 1 consisted of managing post-op pain and swelling, minimizing post-injection muscle attenuation, limiting activities that increase intra-abdominal pressure, and implementing range of motion exercises. Phase 1 exercises included core/pelvic strengthening exercises in quadruped and side-lying and initiation of a walking/bike riding program.
Phase 2 involved gradually improving the patient’s lower extremity strength and neuromuscular control. The goals for this phase were to progress isotonic exercises, improve lower extremity neuromuscular control, begin a jogging program, and gradually return to light functional activities.
Phase 3 focused on increasing overall strength in the sagittal and frontal plane and implementing power production. The goals for this phase were to progress to aggressive and advanced lower extremity and core sagittal plane strengthening, initiate a plyometric and agility program, progress lower extremity balance on multiple uneven surfaces, initiate therapeutic exercises integrating neurocognitive reactive therapy and progress sprinting.
Phase 4 goals were to initiate lower extremity and core strengthening in the transverse plane, progress lower extremity weight training, progress agility, sprinting and ply-
ometric activities, start a jump/landing program, and progress therapeutic exercises with neurocognitive reactive therapy and initiate non-contact practice sport-specific drills.
Phase 5 was the progression of jump/landing program, participation in sports practice, progression of cutting drills, and progression to full sports participation. Full return to sport occurred at six weeks after the injection.
Table 1 summarizes patient-reported and clinical outcomes at initial examination and discharge. The patient demonstrated improved hip and groin self-reported function, strength, range of motion, performance.
All patient reported and psychosocial based outcomes are listed in Table 1 The patient met MCID’s for the HAGOS subscales, as well as the LEFS (MCID = 11.3). The subject achieved their initial patient specific functional goals (kick a soccer ball and pivot while running and compete competitively in soccer and soccer workouts) without pain and limitation set at the initial evaluation. The subject received accolades for offensive player of the year following the completion of the season. He reported no limitation following physical therapy intervention.
The OSPRO-YF questionnaire indicated a positive yellow flag for fear of physical activity at the initial evaluation. Following physical therapy, the patient was negative for fear of physical activity and had met MCID for fear of physical activity (MCID = 14.95). There were no other negative coping or pain susceptibility indicators identified during the initial examination.
All impairment-based outcomes are listed in Table 2. Passive range of motion was measured in flexion, abduction, external and internal rotation. The patient met MDC for hip flexion (8.2°) with a change of 10°, hip abduction (7.3°) with a change of 15°, hip external rotation (7.1°) with a change of 5°, but not internal rotation (7.8°) with a loss of one degree.
The patient demonstrated improvements strength of the hip flexors, abductors, and adductors compared to baseline. The patient met MDC for hip flexion (80.1 N), hip adduction (30.8 N), and hip abduction (35.5 N) with a change of 80.41 N in flexion, 80.41N in adduction, and 133.38 N in abduction. Isokinetic testing conducted at discharge revealed a 3.4% quadriceps deficit involved (right side peak torque = 126.6 ft/lbs) compared to uninvolved (left side peak torque = 131.0 ft/lbs) and 23.7% stronger involved hamstrings (right side peak torque = 91.0 ft/lbs) compared to the uninvolved side (left side peak torque = 73.5 ft/lbs). Limb sym-
*Met MCID/MDC, ^Positive for Psychological Yellow Flag, βNot Established, MCID Minimal Clinically Important Difference, MDC Minimal Detectable Change, HAGOS= The Copenhagen Hip and Groin Outcome Score, OSPRO-YF=Optimal Screening for Prediction of Referral and Outcome Yellow Flag Questionnaire, FABQ-W= Fear Avoidance Belief Questionnaire-Work Scale, FABQ-PA= Fear Avoidance Belief Questionnaire-Physical Activity Scale,TSK-11=Tampa Scale for Kniesiopobia-11, PCS= Pain Catastrophizing Scale, STAI=State Trait Anxiety Inventory, STAXI=State Trait Anger Expression Inventory, PHQ-9= Patient Health Questionnaire-9, PASS 20=Pain Anxiety Symptom Scale, PSEQ=Pain Self Efficacy Questionnaire, SER=Self Efficacy for Rehabilitation, CPAQ=Chronic Pain Acceptance Questionnaire
Table 2. Impairment-based clinical measures
*Met MDC, MDC = Minimal Detectable Change, ROM=Range of Motion, deg=degrees, Kg=kilograms, N=Newtons
metry index of the quadriceps was 96.64% and for hamstring was 118.95%.
Strength Based (Isokinetic) Test at Discharge
Jump Based Performance Tests at Discharge
deg/sec=degree per second, ft/lbs.= foot-pounds, cm= centimeters, Ω=Limb Symmetry Index
STRENGTH AND PERFORMANCE -BASED OUTCOMES AT DISCHARGE
The patient demonstrated an LSI index of 106% for the single leg hop, a LSI of 98% for the triple hop, and a LSI of 101% for the cross over hop (involved > uninvolved). (Table 3)
This case report describes the successful treatment of adductor-related groin pain in a competitive youth soccer player with US-guided PRP injection and multi-phased physical therapy Meaningful improvements in clinical and patient-reported outcomes were noted. These outcomes included improvements in patient-reported outcomes, negative coping indicators such as fear, impairment-based outcomes, and performance-based outcomes. Utilizing US-guided PRP along with appropriate physical therapy may allow athletes to return to sport and complete their season without significant time loss.
Scholten et al., described the utilization of US-guided PRP for the successful treatment of distal rectus abdominis tendinopathy in a lacrosse player, however that patient had a concomitant hip labral tear that was treated with surgery prior to the PRP injection for adductor-related groin pain.8 This case describes the utilization of PRP as a first line intervention followed by a phased intervention physical therapy approach for adductor-related groin pain alone. PRP may allow a window for expedited healing and symptoms improvement allowing for accelerated rehabilitation compared to physical therapy alone or with the administration of a corticosteriod.42‑45 In fact, its reported that physical therapy alone or in conjunction with a corticosteroid injection may take eight weeks before return to sport in acute
groin injuries, and up to six months for chronic strains.46,47
Additionally, research indicating the short- and long-term results of corticosteroid injections in the treatment of groin pain have not been well demonstrated.12,48 The results in of gains in psychosocial outcomes, range of motion, and strength could be due to diminished pain allowing earlier progression of rehabilitation. PRP may be a promising adjunct to physical therapy, allowing accelerated rehabilitation and return to sport.
While the outcomes of this case report demonstrated value of PRP as a treatment adjunct, definitive conclusions cannot be made due to limitations inherent to case reports. Furthermore, it cannot be said definitively that PRP injection was the deciding factor in the successful rehabilitation of this athlete in this case, as physical therapy intervention alone and/or tissue healing time may elicit similar outcomes. Future studies will be necessary to elucidate causation.
US-guided PRP in conjunction with a phased physical therapy program was effective in treating a competitive soccer player with adductor-related groin pain affording resolution of symptoms, improved strength, minimal time loss from competitive play, and successful return to previous level of play US guided PRP warrants further clinical consideration as an alternative intervention in athletes with adductor-related groin pain.
Giorgio Zeppieri Jr , PT, SCS Department of Rehabilitation, University of Florida, Team
Nonsurgical Management of Adductor-related groin pain with Ultrasound-Guided Platelet-Rich Plasma Inj…
Physical Therapist, University of Florida Health
3450 Hull Rd, Gainesville, FL 32607 (USA)
E-mail: zeppig@shands.ufl.edu
SUBJECT CONSENT
The subject was informed prior to treatment that data concerning the case would be submitted for publication.
Submitted: February 26, 2024 CDT, Accepted: June 05, 2024 CDT
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Candela V, De Carli A, Longo UM, et al. Hip and groin pain in soccer players. Joints 2021;7:182-187
2. Mosler AB, Weir A, Eirale C, et al. Epidemiology of time loss groin injuries in a men’s professional football league: A 2-year prospective study of 17 clubs and 606 players. Br J Sports Med. 2018;52:292-297 doi:10.1136/bjsports-2016-097277
3. Werner J, Hagglund M, Walden M, Elkstrand J. UEFA injury study: A prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br J Sports Med 2009;43(13):1036-1040. doi:10.1136/ bjsm.2009.066944
4. Caudil P, Nyland J, Smith C, Yerasimides J, Lach J. Sports hernias: A systematic literature review. Br J Sports Med 2008;42:954-964. doi:10.1136/ bjsm.2008.047373
5. Elattar O, Choi H, MD, Dills VD, Busconi B. Groin injuries (athletic pubalgia) and return to play. Sports Health 2016;8(4):313-323. doi:10.1177/ 1941738116653711
6. Drager J, Rasio J, Newhouse A. Athletic pubalgia (Sports Hernia): Presentation and treatment. Arthroscopy 2020;36(12):2952-2953. doi:10.1016/ j.arthro.2020.09.022
7 Meyers WC, Yoo E, Devon ON, et al. Understanding “sports hernia”(athletic pubalgia): The anatomic and pathophysiologic basis for abdominal and groin pain in athletes. Oper Tech Sports Med 2012;20:33-45. doi:10.1053/j.otsm.2012.03.005
8. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: A case report. PM & R 2015;7:79-83. doi:10.1016/ j.pmrj.2014.08.943
9. LeBlanc KE, LeBlanc KA. Groin pain in athletes. Hernia 2003;7:68-71. doi:10.1007/ s10029-002-0105-x
10. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med 2000;28:2-8. doi:10.1177/ 03635465000280011501
11. Steele P, Annear P, Grove JR. Surgery for posterior wall inguinal deficiency in athletes. J Sci Med Sport 2004;7:415-421. doi:10.1016/S1440-2440(04)80257-3
12. Larson CM. Sports hernia/athletic pubalgia: Evaluation and treatment. Sports health 2014;6(2):139-144. doi:10.1177/1941738114523557
13. Paajanen H, Brinck T, Hermunen H, Airo I. Laparoscopic surgery for chronic groin pain in athletes is more effective than nonoperative treatment: A randomized clinical trial with magnetic resonance imaging of 60 patients with sportsman’s hernia (athletic pubalgia). Surgery. 2011;150(1):99-107 doi:10.1016/j.surg.2011.02.016
14. Kraeutler MJ, Mei-Dan O, Belk JW, Larson CM, Talishinskiy T, Scillia AJ. A systematic review shows high variation in terminology, surgical techniques, preoperative diagnostic measures, and geographic differences in the treatment of athletic pubalgia/ sports hernia/core muscle injury/inguinal disruption. Arthroscopy 2021;37(7):2377-2390. doi:10.1016/ j.arthro.2021.03.049
15. Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-Rich plasma: New performance understandings and therapeutic considerations. Int J Mol Sci 2020;21(20):7794. doi:10.3390/ijms21207794
16. James JSLJ, Ali AK, Pocock PC, et al. Ultrasound guided dry needling and autologous blood injection for patellar tendinosis. Br J Sports Med. 2007;41:518-521. doi:10.1136/bjsm.2006.034686
17 Kon E, Filardo G, Delcogliano M, et al. Plateletrich plasma: New clinical application: A pilot study for treatment of jumper’s knee. Injury 2009;40:598-603. doi:10.1016/j.injury.2008.11.026
18. Sanchez M, Anitua E, Azofra J, Andıa I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet rich fibrin matrices. Am J Sports Med. 2007;35:245-251. doi:10.1177/ 0363546506294078
19. Everts PA, Devilee RJJ, Brown Mahoney C, et al. Exogenous application of platelet-leukocyte gel during open subacromial decompression contributes to improved patient outcome. Eur Surg Res 2008;40:203-210. doi:10.1159/000110862
20. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20–22):1584-1589. doi:10.1080/ 09638280801906081
21. Thorborg K, Hölmich P, Christensen R, Petersen J, Roos EM. The Copenhagen Hip and Groin Outcome Score (HAGOS): development and validation according to the COSMIN checklist. Br J Sports Med. 2011;45(6):478-491. doi:10.1136/bjsm.2010.080937
22. Thorborg K, Kraemer O, Madsen AD, Hölmich P
Patient-reported outcomes within the first year after hip arthroscopy and rehabilitation for femoroacetabular impingement and/or labral injury: the difference between getting better and getting back to normal. Am J Sports Med 2018;46(11):2607-2614. doi:10.1177/ 0363546518786971
23. Kemp JL, Collins NJ, Roos EM, Crossley KM. Psychometric properties of patient-reported outcome measures for hip arthroscopic surgery. Am J Sports Med 2013;41(9):2065-2073. doi:10.1177/ 0363546513494173
24. Thomeé R, Jónasson P, Thorborg K, et al. Crosscultural adaptation to Swedish and validation of the Copenhagen Hip and Groin Outcome Score (HAGOS) for pain, symptoms and physical function in patients with hip and groin disability due to femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):835-842. doi:10.1007/ s00167-013-2721-7
25. Mehta SP, Fulton A, Quach C, Thistle M, Toledo C, Evans NA. Measurement properties of the Lower Extremity Functional Scale: A systematic review J Orthop Sports Phys Ther 2016;46(3):200-216. doi:10.2519/jospt.2016.6165
26. Hefford C, Abbott JH, Arnold R, Baxter GD. The patient-specific functional scale: validity, reliability, and responsiveness in patients with upper extremity musculoskeletal problems. J Orthop Sports Phys Ther 2012;42(2):56-65. doi:10.2519/jospt.2012.3953
27 Zeppieri G Jr, George SZ, Bialosky J, Lentz TA. Patient-centered outcomes: Domain importance predicts health care use following physical therapy P M & R. 2022;14(9):1044-1055. doi:10.1002/ pmrj.12680
28. Lentz TA, Beneciuk JM, Bialosky JE, et al. Development of a yellow flag assessment tool for orthopaedic physical therapists: Results from the optimal screening for prediction of referral and outcome (OSPRO) cohort. J Orthop Sports Phys Ther. 2016;46(5):327-343. doi:10.2519/jospt.2016.6487
29. Thorborg K, Hölmich P Hip- and knee-strength assessments using a hand-held dynamometer with external belt-fixation are inter-tester reliable. Knee Surg Sports Traumatol Arthrosc 2013;21(3):550-555. doi:10.1007/s00167-012-2115-2
30. Feiring DC, Ellenbecker TS, Derscheid GL. Testretest reliability of the biodex isokinetic dynamometer J Orthop Sports Phys Ther 1990;11(7):298-300. doi:10.2519/jospt.1990.11.7.298
31. Thorborg K, Petersen J, Magnusson SP, Hölmich P. Clinical assessment of hip strength using a hand-held dynamometer is reliable. Scand J Med Sci Sports 2010;20(3):493-501. doi:10.1111/ j.1600-0838.2009.00958.x
32. Krause DA, Neuger MD, Lambert KA, Johnson AE, DeVinny HA, Hollman JH. Effects of examiner strength on reliability of hip-strength testing using a handheld dynamometer J Sport Rehabil 2014;23(1):56-64. doi:10.1123/JSR.2012-0070
33. Pua YH, Wrigley TV, Cowan SM, Bennell KL. Intrarater test-retest reliability of hip range of motion and hip muscle strength measurements in persons with hip osteoarthritis. Arch Phys Med Rehabil 2008;89(6):1146-1154. doi:10.1016/ j.apmr.2007.10.028
34. Ellenbecker TS, Ellenbecker GA, Roetert EP, et al. Descriptive profile of hip rotation range of motion in elite tennis players and professional baseball players. Am J Sports Med 207(35):1371-1376. doi:10.1177/ 0363546507300260
35. Prather H, Harris-Hayes M, Hunt DM, et al. Reliability and agreement of hip range of motion and provocative. PM R 2010;2(10):888-895. doi:10.1016/ j.pmrj.2010.05.005
36. Bates K, Zeppieri G, Young C, et al. Preseason lower extremity range of motion, flexibility, and strength in relation to in-season injuries in NCAA division I gymnasts. Phys Sportsmed 2024;52(2):200-206. doi:10.1080/ 00913847.2023.2215775
37 Ore V, Nasic S, Riad J. Lower extremity range of motion and alignment: A reliability and concurrent validity study of goniometric and three-dimensional motion analysis measurement. Heliyon 2020;6:e04713. doi:10.1016/j.heliyon.2020.e04713
38. Zeppieri GJ, Lentz TA, Moser MW. Changes in hip range of motion and strength in collegiate baseball pitchers over the course of a competative season: A pilot study. Int J Sports Phys Ther. 2015;10(4):505-513.
39. Reid A, Birmingham TB, Stratford PW, Alcock GK, Giffin JR. Hop testing provides a reliable and valid outcome measure during rehabilitation after anterior cruciate ligament reconstruction. Phys Ther 2007;87(3):337-349. doi:10.2522/ptj.20060143
40. Hammoud S, Bedi A, Magennis E, Meyers WC, Kelly BT. High incidence of athleticpubalgia symptoms in professional athletes with symptomatic femoroacetabular impingement. Arthroscopy. 2012;28:1388-1395. doi:10.1016/j.arthro.2012.02.024
41. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: A case series. Arthroscopy 2011;27:768-775. doi:10.1016/ j.arthro.2011.01.018
42. Cecerska-Hery´c E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev 2022;64:84-94. doi:10.1016/j.cytogfr.2021.11.003
43. Le ADK, Enweze L, DeBaun MR, Dragoo JL. Platelet-rich plasma. Clin Sports Med 2019;38(10):17-44.
44. Reurink G, Goudswaard GJ, Moen MH, et al. Platelet-Rich plasma injections in acute muscle injury. New Engl J Med. 2014;370(26):2546-2547. doi:10.1056/NEJMc1402340
45. Xu Z, He Z, Shu L, Li X, Ma M, Ye C. IntraArticular platelet-rich plasma combined with hyaluronic acid injection for knee osteoarthritis is superior to platelet-rich plasma or hyaluronic acid alone in inhibiting inflammation and improving pain and function. Arthroscopy 2021;37(3):903-915. doi:10.1016/j.arthro.2020.10.013
46. Morelli V, Weaver V. Groin injuries and groin pain in athletes: Part 1. Prim Care 2005;32(1):163-183. doi:10.1016/j.pop.2004.11.011
47. Holmich P, Uhrskou P, Ulnits L, et al. Effectiveness of active physical training as treatment for longstanding adductor-related groin pain in athletes: Randomised trial. Lancet. 1999;353(9151):439-443. doi:10.1016/S0140-6736(98)03340-6
48. Campbell KJ, Boykin RE, Wijdicks CA, Giphart EJ, LaPrade RF, Philippon MJ. Treatment of a hip capsular injury in a professional soccer player with plateletrich plasma and bone marrow aspirate concentrate therapy. Knee Surg Sports Traumatol Arthrosc 2013;21:1684-1688. doi:10.1007/ s00167-012-2232-y
Download: https://ijspt.scholasticahq.com/article/120209-nonsurgical-management-of-adductor-related-groin-painwith-ultrasound-guided-platelet-rich-plasma-injection-and-physical-therapy-in-a-competitive-socc/attachment/ 232834.docx?auth_token=sX4mbiXU8iW8Z6M74qg3

Published online July 1, 2024:910-922. doi:10.26603/001c.120205
Zacharias
Flore1,2a ,
Karen Hambly
1
, Kyra De Coninck1 , Götz Welsch3,4
1 School of Sport and Exercise Sciences, University of Kent, 2 Medical Department, 1. FC Magdeburg, 3 UKE-Athleticum, University Medical Center Hamburg-Eppendorf, 4 Department of Trauma and Orthopaedic Surgery, University Medical Center Hamburg-Eppendorf
Keywords: lateral ankle sprains, rehabilitation algorithm, professional football (soccer), decision-making, progression https://doi.org/10.26603/001c.120205
Lateral ankle sprain (LAS) is one of the most common types of injury in professional football (soccer) players with high risk of recurrence. The rehabilitation after LAS in professional football players is often still time-based and relies on anecdotal experience of clinicans. There is still a lack of utilization of criteria-based rehabilitation concepts after LAS in professional football.
The aims of this clinical commentary are (1) to critically discuss the need for criteria-based rehabilitation concepts after LAS in professional football players, (2) to highlight the current lack of these approaches and (3) to present a novel clinical guideline-based rehabilitation algorithm.
Short time-loss (15 days) and high recurrence rate (17%) raise the question of trivialization of LAS in professional football. Despite consequences for many stakeholders involved (players, teams, clubs, insurers), there is still a lack of of criteria-based, step-by-step approaches.
The use of a criteria-based rehabilitation approach might reduce the high recurrence rate after LAS in professional football players and will lead, in turn, to increased long-term player availability Practical experiences of he authors demonstrate the feasibility of such an approach. The effectiveness of this novel rehabilitation algorithm remains to be evaluated in future studies.
Level of Evidence: 5
Injury to the ankle is considered to be the third most common type of injury in elite football (soccer) players.1 Fourteen to eighteen percent of all injuries occurring in professional football affect the ankle.2‑4 The ligamentous apparatus is the most frequently injured anatomical structure of the ankle with 67-81% of all ankle injuries affecting the lateral ligament complex (LLC). With more than three fourths of ligament injuries affecting the lateral ligament complex,5,6 the lateral ankle sprain (LAS) is the most common isolated type of ankle injury.6 Up to 40% of all athletes have residual symptoms and develop chronic impairments after the initial LAS.5,7
Corresponding author:
Zacharias Flore University of Kent, School of Sport and Exercise Sciences Kapellenstraße 8a 39104 Magdeburg, Germany zf43@kent.ac.uk +4915203090096 a
Rehabilitation of LAS is often conducted in a time-based manner, with a reliance on clinicians’ anecdotal experience. The American Physical Therapy Association (APTA) appointed an expert panel to review the literature and identify recommendations and updates on the 2013 Clinical Practice Guidelines.8,9 The review8 collated and updated ankle injury management recommendations and their appropriate outcome measures. Contrary to other types of injuries (e.g. groin, Anterior Cruciate Ligament [ACL]), there are still no evidence-based step-by-step approaches to rehabilitation after LAS.10‑13 Individual recommendations for the rehabilitation of ankle sprain injuries14 and broad consensus on their implementation do exist,15,16 but there is no published consensus that combines all recommen-
dations into an evidence-based step-by-step approach.10 First return to competition (RTC) recommendations based on expert opinions exist,16‑18 but there are no rehabilitation concepts that provide tests and criteria for progression within the rehabilitation phase. This lack of standardized rehabilitation approaches seems all the more surprising given the high prevalence, incidence, and recurrence rate of 6% up to 41% of LAS in professional football players,6,19 especially since the idea of criteria-based rehabilitation has already been established for rehabilitation after other types of injury (including ACL).11
Premature RTC and inadequate rehabilitation are considered to be one of the most important risk factors for a recurrent ankle sprain injury 16 High incidence and recurrence rates cause consequences for many stakeholders involved (players, teams, clubs, insurers) in terms of personal athletic development, the club´s financial burden, and treatment costs. A comprehensive testing and criteriabased approach aims to optimize the rehabilitation process while achieving the overall goal of reducing the recurrence rate of LAS in professional football players.
For this reason, the authors have developed a rehabilitation algorithm (Supplementary Material 1) that determines progression within rehabilitation by ensuring athletes pass specific standardized clinical examinations, performance tests, and achieve a specific questionnaire score (Ankle Function Score, AFS) defined for each rehabilitation level. The most current CPG assessments have been integrated into a step-by-step approach within the acute and subacute phase. However, there is still a lack of a CPG or recommended assessments within the ongoing RTS phases until RTC.
The primary aim of this clinical commentary is to critically discuss the need for criteria-based rehabilitation concepts after LAS in professional football. The secondary aim is to highlight the lack of theses approaches. The third aim is to present a criteria-based rehabilitation algorithm following lateral ankle sprains in professional football players through the rehabilitation phase until final clearance for return to competition.
While criteria-based approaches have been developed in recent decades for different body regions (knee, groin, thigh) and various types of injury (ACL, adductor injuries),11,12 rehabilitation after LAS commonly remains time-based and relies on the anecdotal experience of clinicians.13,16 Missing criteria for phase-sensitive progression within the rehabilitation phase as well as missing final test criteria for release to RTC may increase the risk of premature sports participation.13 The average time-loss after LAS in elite football players is 15 days and is thus often below the recovery time required for physiological ligament healing.20,21 This means that elite football players may return to competition too soon with incompletely healed ligaments.22 Premature RTC is considered to be one of the highest risk factors for re-injury.16 The recurrence rate after LAS in elite
football players averages 17%.20 which is one of the highest recurrence rates of all sports injuries.13,16 The short time-loss (15 days) and high recurrence rate (17%) raise the question of trivialization of LAS injuries in elite footballers. A criteria-based rehabilitation program could manage rehabilitation and get the athlete back on the pitch as safely as possible, rather than as quickly as possible. The overall goal should be to lower the recurrence rate and reduce the risk of possible long-term consequences.13 Tassignon13 could not detect any study that included a criteria-based rehabilitation concept. There is a need to develop phase-sensitive and -specific multifactorial assessments and decision-making models.10,13 LAS is still trivialised and injured athletes are expected to return to competition as quickly as possible. The scientific evaluation of these concepts, in particular the objective assessment of their effectiveness in reduced risk of reinjury, could bring about a shift in coaches’ thinking regarding the risk of long-term sporting and health consequences for the athlete (Supplementary Material 2).
The authors have developed a criteria-based rehabilitation algorithm based on specific test and progression criteria. Due to the need for and the lack of criteria-based rehabilitation concepts after LAS in professional football, the algorithm was developed in 2017 The methology of its development is presented in supplemental material (Supplementary Material 3). This algorithm is presented in full step-by-step detail in the supplementary material. Progression through rehabilitation levels is controlled by passing predefined assessments. Superordinate domains which should be included in test batteries for decision-making of RTC based on expert opinions and consensus are already described.15,16,18 However, there is still a lack of tests and criteria to guide rehabilitation. Tassignon13 recommends integrating phase-sensitive domains in a dynamic rehabilitation model. Specific tests and criteria for secure phase transition are defined from a multitude of described assessments (Supplementary Material 1), which potentially correspond to the biomechanical requirements of the subsequent rehabilitation level and take into account the status of physiological ligament healing. The patient’s perspective is also taken into account by integrating a patient reported outcome/questionnaire (AFS) and a standardized clinical examination based on recommended clinical examination standards (ROM, strength, manual joint tests including the Anterior Drawer Test [ADT], Talar Tilt Test [TLT]; circumferential measurement). Players are progressed by integrating phase-specific functional performance tests (FPT). The FPTs represent the expected biomechanical requirements of the subsequent rehabilitation level and should thus ensure safe rehabilitation training (Supplementary Material 4 and 5). With this approach, the authors combine the individual assessments for the first time in an arrangement under progressive aspects and under consideration of physiological ligament healing. The rehabilitation algorithm is an evidence-based concept. It takes into account the current scientific recommendations derived from clinical practice guidelines23 and considers the application in everyday practice in professional football.
OF A NOVEL CRITERIA-BASED REHABILITATION ALGORITHM
REHABILITATION ALGORITHM WITHIN THE POSTINJURY PYRAMID
The rehabilitation algorithm is a concept consisting of four levels (Figure 1). As part of a post-injury phase (rehabilitation process), it forms the lowermost differentiated part of a post-injury pyramid (Figure 2). The prerequisite for progressing to a subsequent rehabilitation level is passing three assessments (achieving a level-dependent score in the patient reported outcome measure, passing a standardised clinical examination, and passing performance tests). The athlete remains at a rehabilitation level until each assessment for progression to the next level is passed.
The entire rehabilitation phase consists of four macrophases (Return to Activity [RTA], Return to Sports [RTS], Return to Play [RTP], Return to Competition [RTC]). In the RTA phase, the greatest differentiation takes place in the entire rehabilitation process, taking into account the timedependent wound healing phases. Sport-specific (football) training forms are the focus in the RTS phase. After the integration into team training (RTP), the athlete is released for unrestricted participation in competition (RTC).
Within the RTA level, each individual test battery for the phase transition and training form is in itself progressively structured. The approach respects the anatomical nature and biomechanical alignment of the ligament structures, the load tolerance at the respective stages of wound healing, and the load stimuli necessary for optimal healing (e.g. collagen synthesis through dosed tensile stimuli).
The integration of three test tools (the AFS, clinical examination, performance tests) may help to ensure readiness for phase transition from multiple perspectives. The integration of a questionnaire (patient reported outcome measure) is generally recommended as a complement to performance testing.11,13,24 In so doing, subjective feedback is provided by the patient which can support the decisionmaking process alongside objective functional tests.25 A validated questionnaire can bridge the gap between subjective patient assessment and objective measurement parameters in the RTS process and should be part of a criteriaguided rehabilitation process.11 Integrating a standardized clinical examination ensures the testing of clinical criteria (range of motion (ROM), joint stability, signs of inflammation, muscle function tests, assessment of swelling) and enables progress monitoring during rehabilitation. Performance tests (proprioception and hop tests) are established standards for guiding rehabilitation and for final testing of readiness for RTC.26‑28 Hop tests are frequently used performance measures.29‑31 Functional performace tests are practical, cost-efficient, and can be applied quickly and easily in almost any clinical setting without much equipment.29,30,32,33
One method for patient-centred assessment of readiness to return to sport is the use of patient reported outcomes /questionnaires.11,25 Most questionnaires only test the athlete’s physical or psychological readiness at the end of rehabilitation before the RTC. Hence, they are outcome measures and not designed for process evaluation.34 The AFS is a questionnaire consisting of five items (pain, instability, weight bearing, swelling, gait pattern). In total 100 points can be scored.35 Because of its scoring system, the AFS may enable objective and comparable sustainable progress monitoring to control the progression within rehabilitation. Therefore, the AFS is evaluative36 and can be used as an additional tool to the clinical examination and performance tests to assist in guiding and evaluating the rehabilitation process.37 The AFS is simple to use, quick to complete, and to evaluate. Therefore, it is suitable for daily practice, especially in competitive sports, to manage rehabilitation and process assessment.37
Passing a standardized clinical examination is an essential component when it comes to making decisions on progression to a subsequent rehabilitation phase. Clinical Practice Guidelines find moderate evidence to classify LAS based on clinical findings of function, ligament laxity, pain, swelling, hemorrhaging, point tenderness and ankle motion.8 The clinical examination consists of manual ankle tests: Anterior Drawer Test (ADT) and Talar Tilt Test (TLT), manual muscle function tests [MFT], circumference measurement, as well as range of motion (ROM) assessments. The ADT and TLT are specific stability tests to examine the integrity and mechanical stability of the lateral ligaments. Moreover, they are also used in clinical examinations to monitor progress7,28 with high sensitivity from post injury day five.15,38 Muscle function tests (MFT) are suitable for determining muscle strength status and can also be used to monitor progress toward regaining strength abilities.39 They are standard tests of isometric muscle strength.28,40 A near full ROM is a prerequisite for subsequent training and movement sequences. As ROM restrictions (especially in the anterior direction)41,42 remain one of the most common deficits after LAS gradual improvement of ROM is an essential part of the clinical progression. Side to side difference in ROM should be less than 10 degrees of dorsiflexion to pass the clinical examination for Levels 1 and 2. Furthermore, ROM should be the same compared to the non-injured ankle to pass the criteria for progression to the ongoing levels. ROM is often hindered by persistent swelling. Swelling can negatively affect muscle, joint, and proprioception function18,43 and should be controlled especially in the early functional phase. The swelling should not be more than +1% compared to the previous measurement to check possible irritant reactions.
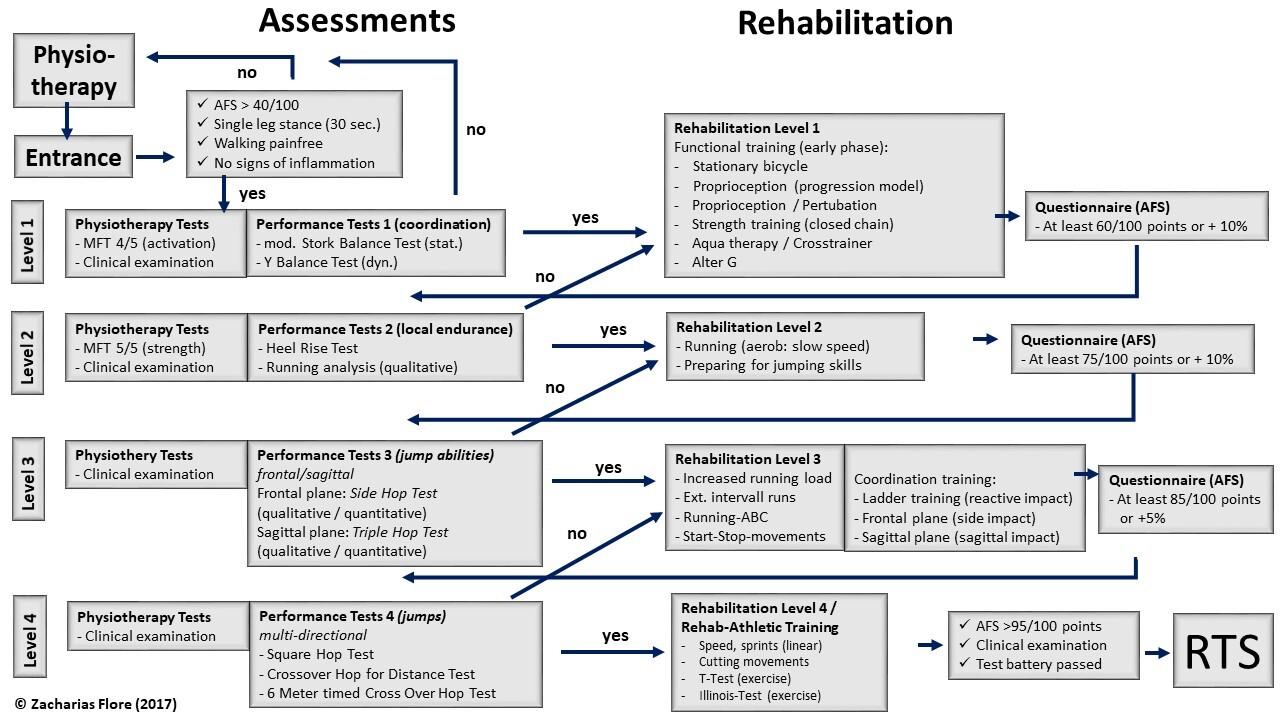
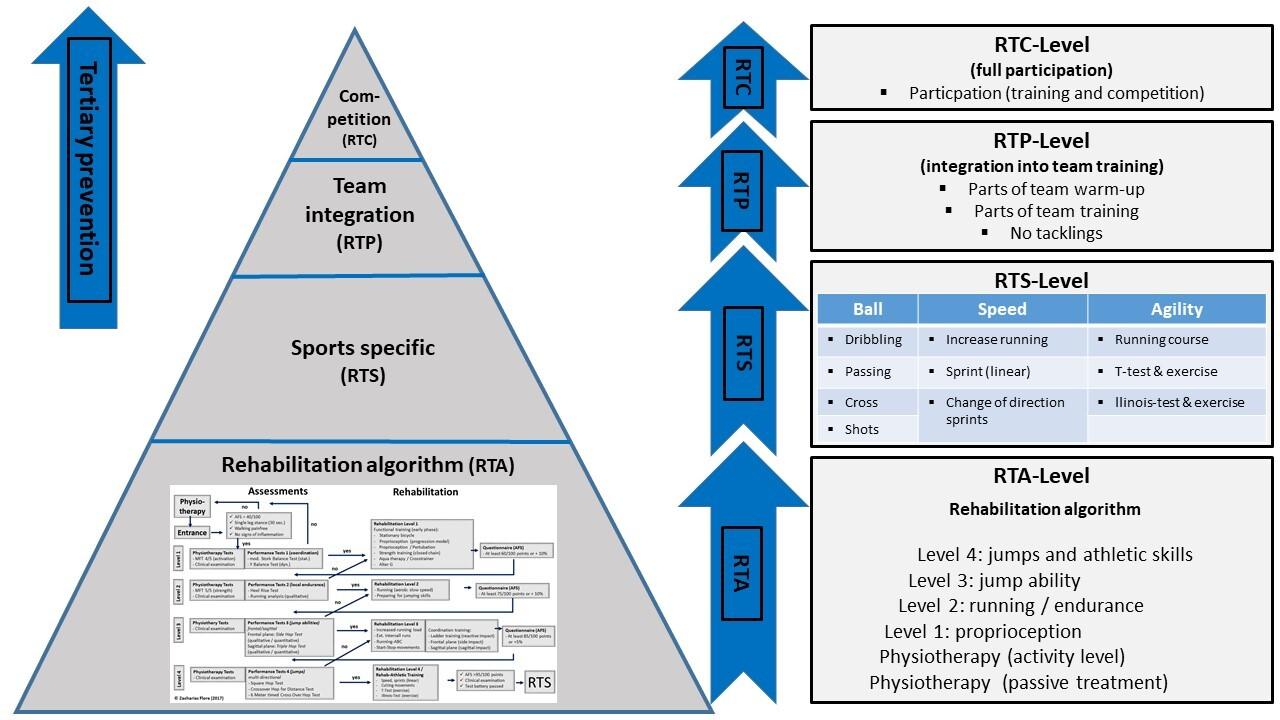
Figure 2. Post injury pyramid
FUNCTIONAL PERFORMANCE TESTS
Functional performance tests (FPT), especially proprioception and hop tests, are established standards for assessing rehabilitation after sports injuries.29,31,44 FPT can objectively guide progression through rehabilitation and determine release for unrestricted participation.32,33,45 In the proposed algorithm, tests are arranged progressively, taking into account the phases of wound healing, loading capacities, and objectives of the training exercises of the subordinate training levels. Level 1 aims to train proprioceptive skills (sensory perception, afferentiation, “joint sense”) while avoiding biomechanical impulse stress on the lateral ligament complex. Thus, passing proprioception tests including the static modified Stork Balance Test and dynamic Y-Balance Test is a prerequisite for release into Level 1. The aim of Level 2 is a return to running. The heel rise test and qualitative running analysis are part of the functional testing for entering the second level. Taking into account the time-dependent progression of wound healing and integrity of the ligaments, the performance tests in Level 3 are increasingly more dynamic and include the Side Hop Test (SHT) and Triple Hop Test (THD). Passing highly dynamic multi-directional hop tests (Square Hop Test, Crossover Hop Test, modified 6m timed Crossover) is required for release into Level 4. The test sequence is designed to be progressive so that combined sagittal and frontal hops are performed first under high control (Square Hop Test), progressing to reduced control (Crossover Hop Test), and then under time pressure conditions (modified 6m timed Crossover). Because of familiarization aspects, the testing of high-dynamic jump tests in Levels 3 and 4 is initially conducted qualitatively. In this way, the quality of movement and the pain response can be assessed prior to
quantitative testing and, if necessary, a potentially damaging/injurious quantitative test can be avoided. Passing the functional performance tests is determined by a Limb Symmetry Index (LSI) of the injured leg greater than 90% compared to the uninjured leg. The LSI is a useful and easy to perform assessment of lower limb function using the uninjured limb as a control.
29
DISCUSSION ON SPECIFIC COMPONENTS OF THE REHABILITATION ALGORITHM
CLINICAL PRACTICE GUIDELINES WITHIN THE ALGORITHM
CPGs can be used to integrate the best evidence into clinical rehabilitation of LAS as they are an important move toward developing specific rehabilitation approaches. Relevant tests and assessments were used as progression criteria. While the CPGs summarize the best evidence of clinical assessments, the rehabilitation algorithm brings this best evidence together in how it is practically applied. The table (Supplementary Material 6) summarizes similarities and differences between the most recent CPG (2021) and the rehabilitation algorithm presented herein, and demonstrates the justification for it´s integration.
The AFS is an evaluative instrument that can guide the course of rehabilitation.36,37 Specific benchmarks were set at each phase transition (40, 60, 75, 85, 95) based on our experience in accordance with the literature. The AFS was not explicitly developed for process evaluation after LAS injuries in elite football, but it is particularly suitable for
Table 1. Progression criteria and training exercises (examples, SM_ Fig. 3+4)
Level
1: Proprioception
• AFS>40/100
• Pass the clinical examination
• Pass proprioceptive performance tests (modified Stork Balance Test; Y-Balance Test)
2: Running
3: Jump abilities
4: athletic skills (rehabilitation)
• AFS>60/100
• Pass the clinical examination
• Pass Heel Rise Test and qualitative running analysis
• AFS>75/100
• Pass the clinical examination
• Pass hop tests (Side Hop Test; Triple Hop Test)
• AFS>85/100
• Pass the clinical examination
• Pass hop tests (Square Hop Test; Crossover Hop Test; mod. 6m-timed Crossover Hop Test)
RTS: footballspecific
RTP: reintegration (team training)
RTC: team training
• AFS>95/100
• Pass the test battery
• lack of criteria; experienced-based; subjective
• VBG test battery
• Lack of evidence
• Weight bearing exercises (proprioceptive); progession model; principles of motor learning (e.g. external focus strategies)
• Closed-chain strength training (e.g. peroneal)
• Stationary bicycle
• Crosstrainer
• Alter G
• Linear running
• Progression in linear running speed
• Hops and bounds (progression)
• Ladder training (short reactive impacts)
• Extensive interval running
• Athletic skills
• Multidirectional movement patterns
• Sprinting
• High-intensity runs
• Cutting, change of direction
• Dribbling, passing, shooting from the short to the long distance (progression model)
• Re-integration into team training
• Team warm-up
• Training forms without opponents
• No tackling (protected player)
• Team training
Only new exercises for each level added. Exercises from previous levels can be applied. AFS, Ankle Function Score; RTP, Return to Play; RTS, Return to Sports; VBG,Verwaltungs Berufsgenossenschaft (German elite sports insurance)
daily use in professional sports. The authors recommend the evaluation of the use of the AFS as a prognostic and evaluative tool for process control after LAS injury in elite football players.
A standardized clinical examination consists of both ROM and muscle strength assessments and measurements of effusion and laxity. ROM is often limited after LAS injury and should be restored within two weeks post-injury 46,47 Manual therapy is recommended to restore ROM.48 Unrestricted ROM is required for testing and training.
The integration of muscle strength tests is recommended due to impairments in muscle function (strength, reaction time), especially of the peroneal muscles.38,49 Reduced muscle functioning of the peroneus muscles may affect the risk of re-injury due to its ankle protection function.38 It is recommended to test muscle function with a dynamometer in order to produce objective outcomes.15,50 However, manual muscle testing procedures are used by the authors for practical reasons. Dynamometers are expensive and not available in every clinical setting. A manual muscle test can offer an alternative to objective measurement of
progression.39 Various cut-offs are proposed to check different strength abilities at each level.
In addition, the ADT and TLT are used to assess ligament integration, stability, and laxity of the ankle. The ADT and TLT are manual ankle tests to assess Anterior Talo-Fibular Ligament (ATFL) [ADT] and Calcaneo-Fibular Ligament (CFL) [TLT], the most commonly injured ligaments in the ankle joint.7,13 The ADT and TLT are recommended to be integrated in an ankle assessment7,28 with specifity of 0.67-1.00 and sensitivity of 0.50-0.97, respectively 15,38, 51‑53 Current clinical guidelines recommend the use of the Reverse anterolateral drawer test (RALDT) and anterolateral talar palpation in addition to the ADT. Even though it has limited accuracy and reliability the ADT is still one of the most commonly utilized tests for ankle sprain assessment.8 The ADT shows the most accuracy when assessed five days post injury 15,38 For this reason, the authors are integrating ADT and TLT for the first time after the acute phase as a complementary assessment tool for transition to Level 1. The authors use a (+) system to objectively assess the laxity during the rehabilitation progression similar to Johnson,54 based on recommendations of grading of Rammelt.55
PERFORMANCE TESTS (FPT)
The integration of functional performance tests (FPT) to assess lower limb function after injury has been recommended elsewhere.29,31‑33,45,56 Functional performance tests are aimed at the basic (fundamental) requirements that an athlete must meet in order to perform their sports.26 Noyes56 described the principle of functional rehabilitation using a jump test battery in the early 1990s. Since then, a variety of functional tests have been developed and evaluated.
Proprioception tests assess general somato-sensory abilities (postural control, balance), while hop tests assess motor control in a more dynamic (multidirectional) environment. While Read52 describes the general comparability of test results due to inconsistent implementation and standards, Davies57 criticizes the ability of individual jump tests to measure performance and outcome of rehabilitation and refers to the lack of biomechanical evaluation studies.57,58 Kotsifaki investigated the biomechanical loads in different joints for common hop tests which is fundamental work to specify the inclusion of hop tests in specific rehabilitation programs due to biomechanical loads which affect individual joints.59,60 Future studies will have to evaluate which loads or directions affect the ankle and its lateral ligament complex in the tests that are used in this algorithm, and whether they exceed the current load capacity (status of healing).
In this algorighm, FPTs are mostly used as criteria for a final test battery at the end of rehabilitation as a decision aid for clearance to RTC.17 The use of phase-sensitive FPTs lacks or phase-sensitive tests have not been evaluated in terms of their phase-specific use (sensitivity; specifity).13
The authors integrate phase-specific FPTs that test the biomechanical requirements of the rehabilitation level to be entered, taking into account time-dependent physiological healing. The training forms of the rehabilitation levels also follow the principle of physiological healing. The tests check the ability to perform the upcoming training skills in advance to ensure safe training.
For example, proprioception tests in Level 1 check somato-sensory abilities. These abilities are fundamental and should be restored as soon as possible for the further course of rehabilitation.14 Proprioception testing and training are low-impact and can therefore be included early in functional training.
Level 2 marks the transition from closed-chain training (e.g. weight-bearing) to open-chain running training. The HRT is a closed-chain test for assessing the calf-muscle strength and endurance61 whilst the qualitative running analysis assesses the ability to run in an open-chain manner. Running is a fundamental movement pattern and is therefore an essential part (milestone) of rehabilitation. A qualitative running analysis is recommended to assess lower limb injury function11,15 and therefore integrated into the rehabilitation algorithm.
The authors integrate several hop tests in Levels 3 and 4. These are valid individually to assess the management of lower limb injuries with a reliability and specificity of
0.66-0.97 and 0.80-0.92 respectively 26,30,58,62 The hop direction is chronological from frontal (SHT), to sagittal (THD), to multidirectional under progressing conditions. The arrangement of the hop tests takes into account the anatomical location of the most frequently injured ligament structures (ATFL, CFL) of the ankle as well as the status of physiological healing.63
Some of the utilzed FPTs are modified. These are intended to improve the tests for the ankle sprain rehabilitation setting. A modification of FPTs in terms of their targeted use is recommended by Caffrey.45
The LSI is a simple method to obtain a prediction of the side-to-side difference in the lower limbs in FPTs. Several authors emphasize the advantages of the methodology in the clinical setting, as it is practical and can be used without software calculation.29,64 The LSI is increasingly discussed critically as it underestimates potential performance deficits, evokes questions about the accuracy of the methology and overlooks “true deficits” in supposedly easy performance tests.44 The use of LSI for quantifying the results of performance tests has mainly been evaluated in ACL patients or healthy individuals. Few LSI results are reported for performance testing after ankle injuries, which highlights both the need for normative data for performance testing in elite football players after ankle injuries and their LSI results. The authors integrate the LSI into the assessment of the performance tests, especially because of its applicability as a simple tool to quantify the test results, while being aware (and mindful) of its limitations. Ultimately, the use of LSI remains debatable for assessing lateral differences in the lower limbs after injury
Arundale65 has recommended a return to ball training at the earliest possible point of time for football players. Returning to the ball after injury can have a motivating effect and support the rehabilitation process.65 However, it must be considered that not every injured body site can be evaluated in the same way: As football is played with the feet, the ball training directly impacts the injured structure. Therefore, special attention should be devoted to a patient wth an ankle sprain during ball training. Impacts of several thousand Newtons can be generated on the foot/ankle during kicks.66‑68 Incorrectly hit balls, especially under fatigued conditions at the end of a training session, can hyperextend the capsular ligamentous apparatus and lead to renewed microtrauma. The authors suggest using different ball types (e.g. volley ball, soft ball) to allow ball training without affecting the healing status negatively. Impacts on the foot/ankle during kicking have been described in several studies.66‑68 So far, there are no step-by-step approaches for specific types of injury taking into account biomechanical impacts.65 For this reason, we recommend orientation to an IKP with attention to clinical responses after rehabilitation training. This makes the standardized clinical examination, which is also part of the rehabilitation algorithm
for this reason, all the more important. The authors view a too-soon return to ball training after LAS injury critically.
While the rehabilitation algorithm can steer the rehabilitation in the acute and sub-acute phase (RTA level) well through its differentiated approach by phase-sensitive testing, taking into account the temporal wound healing phases and load stability of the tissue and ligaments, the standardization of the rehabilitation during the RTS phase is made significantly more difficult by the start of ball training. Currently, there are no football-specific (kicking) tests, nor criteria for football-specific stress progression. Therefore, rehabilitation from this point on until reintegration into team training (RTC) remains anecdotal or experiencebased despite all efforts to make it as objective as possible. The difficulty of objectification beyond this phase is made evident by the lack of Clinical Practice Guidelines beyond the subacute phase.8 Taking into account biomechanical impacts and their integration into a progressive rehabilitation program, kicking test batteries could be an important component of future RTC concepts and could close the currently existing gap between the subacute phase (RTA/RTS level) and the return to unrestricted team training (RTC).
The high recurrence rate (17%) with short time-loss (15 days) after LAS in elite football players raises the question
of trivialization and highlights the need for criteria-based rehabilitation concepts rather than traditional time-based approaches. There is still a lack of criteria-based step-bystep approaches to guide rehabilitation. However, CPGs lack sport-specific tests and assessments especially in the subacute and ongoing phase for RTC decision-making. The presented rehabilitation algorithm is the first to attempt to correlate individual assessments and combine them into a self-contained progressive approach taking into account evidence-based CPGs. Early utilization of this algorithm demonstrate its feasibility. The extent to which this rehabilitation algorithm can sustainably reduce the recurrence rate after LAS in elite football players remains to be evaluated in future studies.
Zacharias Flore University of Kent, School of Sport and Exercise Sciences Kapellenstraße 8a 39104 Magdeburg, Germany zf43@kent.ac.uk +4915203090096
Submitted: January 22, 2024 CDT, Accepted: May 20, 2024 CDT
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. López-Valenciano A, Ruiz-Pérez I, Garcia-Gómez A, et al. Epidemiology of injuries in professional football: a systematic review and meta-analysis. Br J Sports Med 2020;54(12):711-718. doi:10.1136/ bjsports-2018-099577
2. Ekstrand J, Hägglund M, Waldén M. Injury incidence and injury patterns in professional football: the UEFA injury study Br J Sports Med 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582
3. Hawkins RD, Fuller CW A prospective epidemiological study of injuries in four English professional football clubs. Br J Sports Med. 1999;33(3):196. doi:10.1136/bjsm.33.3.196
4. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med 2001;29:426-430. doi:10.1177/ 03635465010290040701
5. D’Hooghe P, Cruz F, Alkhelaifi K. Return to play after a lateral ligament ankle sprain. Curr Rev Musculoskelet Med 2020;13(3):281-288. doi:10.1007/ s12178-020-09631-1
6. Waldén M, Hägglund M, Ekstrand J. Time-trends and circumstances surrounding ankle injuries in men’s professional football: an 11-year follow-up of the UEFA Champions League injury study. Br J Sports Med 2013;47(12):748-753. doi:10.1136/ bjsports-2013-092223
7. Fong DT, Chan YY, Mok KM, Yung PS, Chan KM. Understanding acute ankle ligamentous sprain injury in sports. Sports Med Arthrosc Rehabil Ther Technol 2009;1(14):14. doi:10.1186/1758-2555-1-14
8. Martin RL, Davenport TE, Fraser JJ, et al. Ankle stability and movement coordination impairments: Lateral ankle ligament sprains Revision 2021. J Orthop Sports Phys Ther 2021;51(4):CPG1-CPG80. doi:10.2519/jospt.2021.0302
9. Martin RL, Davenport TE, Paulseth S, Wukich DK, Godges JJ, Orthopaedic Section American Physical Therapy Association. Ankle stability and movement coordination impairments: ankle ligament sprains. J Orthop Sports Phys Ther 2013;43(9):A1-A40. doi:10.2519/jospt.2013.0305
10. Gaddi D, Mosca A, Piatti M, et al. Acute Ankle sprain management: An umbrella review of systematic reviews. Front Med (Lausanne) 2022;9:868474. doi:10.3389/fmed.2022.868474
11. Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE. Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther 2006;36(6):385-402. doi:10.2519/ jospt.2006.2222
12. Serner A, Weir A, Tol JL, et al. Return to sport after criteria-based rehabilitation of acute adductor injuries in male athletes: A prospective cohort study. Orthopaedic Journal of Sports Medicine 2020;8(1):12. doi:10.1177/2325967119897247
13. Tassignon B, Verschueren J, Delahunt E, et al. Criteria-based return to sport decision-making following lateral ankle sprain injury: a systematic review and narrative synthesis. Sports Med. 2019;49(4):601-619. doi:10.1007/s40279-019-01071-3
14. Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Athl Train 2002;37(4):413-429.
15. Delahunt E, Bleakley CM, Bossard DS, et al. Clinical assessment of acute lateral ankle sprain injuries (ROAST): 2019 consensus statement and recommendations of the International Ankle Consortium. Br J Sports Med. 2018;52(20):1304-1310. doi:10.1136/bjsports-2017-098885
16. Wikstrom EA, Mueller C, Cain MS. Lack of consensus on return-to-sport criteria following lateral ankle sprain: A systematic review of expert opinions. J Sport Rehabil 2020;29(2):231-237 doi:10.1123/jsr.2019-0038
17. Bloch H, Klein C, Kühn N, Luig P. Return to Competition – Test Manual for Assessment of the Ability to Play after an Acute Lateral Ankle Sprain Injury. VBG; 2019.
18. Smith MD, Vicenzino B, Bahr R, et al. Return to sport decisions after an acute lateral ankle sprain injury: introducing the PAASS framework-an international multidisciplinary consensus. Br J Sports Med. 2021;55(22):1270-1276. doi:10.1136/ bjsports-2021-104087
19. Amer H, Mohamed S. Prevalence and risk factors of ankle sprain among male soccer players in Tabuk, Saudi Arabia: A cross-sectional study. The Open Sports Sciences Journal 2020;13(19):27-33. doi:10.2174/1875399X02013010027
20. Flore Z, Hambly K, De Coninck K, Welsch G. Time-loss and recurrence of lateral ligament ankle sprains in male elite football: A systematic review and meta-analysis. Scand J Med Sci Sports. 2022;32(12):1690-1709. doi:10.1111/sms.14217
21. Houglum P Soft tissue healing and its impact on rehabilitation. J Sport Rehabil 1992;1(1):19-39. doi:10.1123/jsr.1.1.19
22. Robles-Palazón FJ, López-Valenciano A, De Ste Croix M, et al. Epidemiology of injuries in male and female youth football players: A systematic review and meta-analysis. J Sport Health Sci 2022;11(6):681-695. doi:10.1016/j.jshs.2021.10.002
23. Picot B, Hardy A, Terrier R, Tassignon B, Lopes R, Fourchet F. Which functional tests and self-reported questionnaires can help clinicians make valid return to sport decisions in patients with chronic ankle instability? A narrative review and expert opinion. Front Sports Act Living 2022;4:902886. doi:10.3389/ fspor.2022.902886
24. Richie DH, Izadi FE. Return to play after an ankle sprain: guidelines for the podiatric physician. Clin Podiatr Med Surg. 2015;32(2):195-215. doi:10.1016/ j.cpm.2014.11.003
25. Kaminski TW, Hertel J, Amendola N, Manske. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train 2013;48(4):528-545. doi:10.4085/1062-6050-48.4.02
26. Hamilton RT, Shultz SJ, Schmitz RJ, Perrin DH. Triple-hop distance as a valid predictor of lower limb strength and power. J Athl Train. 2008;43(2):144-151. doi:10.4085/1062-6050-43.2.144
27 Manske R, Reiman M. Functional performance testing for power and return to sports. Sports Health. 2013;5(3):244-250. doi:10.1177/1941738113479925
28. Yoshida M, Taniguchi K, Katayose M. Analysis of muscle activity and ankle joint movement during the side-hop test. J Strength Cond Res. 2011;25(8):2255-2264. doi:10.1519/ JSC.0b013e3181ec86d5
29. Clark NC. Functional performance testing following knee ligament injury Physical Therapy in Sport 2001;2(2):91-105. doi:10.1054/ptsp.2001.0035
30. Logerstedt DS, Snyder-Mackler L, Ritter RC, Axe MJ, Godges JJ, Orthopaedic Section of the American Physical Therapist Association. Knee stability and movement coordination impairments: knee ligament sprain. J Orthop Sports Phys Ther 2010;40(4):A1-A37 doi:10.2519/jospt.2010.0303
31. Vereijken A, van Trijffel E, Aerts I, Tassignon B, Verschueren J, Meeusen R. The non-injured leg can be used as a reference for the injured leg in singlelegged hop tests. Int J Sports Phys Ther. 2021;16(4):1052-1066. doi:10.26603/001c.25758
32. Bolgla LA, Keskula DR. Reliability of lower extremity functional performance tests. J Orthop Sports Phys Ther. 1997;26(3):138-142. doi:10.2519/ jospt.1997.26.3.138
33. Reid A, Birmingham TB, Stratford PW, Alcock GK, Giffin JR. Hop testing provides a reliable and valid outcome measure during rehabilitation after anterior cruciate ligament reconstruction. Phys Ther 2007;87(3):337-349. doi:10.2522/ptj.20060143
34. van der Wees P, Hendriks E, van Beers H, van Rijn R, Dekker J, de Bie R. Validity and responsiveness of the ankle function score after acute ankle injury. Scand J Med Sci Sports 2012;22(2):170-174. doi:10.1111/j.1600-0838.2010.01243.x
35. de Bie RA, de Vet HC, van den Wildenberg FA, Lenssen T, Knipschild PG. The prognosis of ankle sprains. Int J Sports Med 1997;18(4):285-289. doi:10.1055/s-2007-972635
36. Züst et al. Sprunggelenksinstabilität. Rehabilitation. Rückkehr zum Sport. In: GOTSExpertenmeeting: Sprunggelenksinstabilität. ; 2012:79-84. https://sport-ortho.de/files/Bilder-undDokumente/Dokumente/GOTSExpertenmeeting%202012_PE.pdf
37 van der Wees PJ. Evaluation of Evidence-Based Clinical Guidelines in Physical Therapy : Ankle Sprain as Case Example. Doctoral Thesis, Maastricht University Datawyse / Universitaire Pers Maastricht; 2009. doi:10.26481/dis.20090910pw
38. Halabchi F, Hassabi M. Acute ankle sprain in athletes: Clinical aspects and algorithmic approach. World J Orthop 2020;11(12):534-558. doi:10.5312/ wjo.v11.i12.534
39. Wieben K. Muskelfunktion. Prüfung Und Klinische Bedeutung 5. Auflage. Thieme Verlag
40. Lunsford BR, Perry J. The standing heel-rise test for ankle plantar flexion: criterion for normal. Phys Ther 1995;75(8):694-698. doi:10.1093/ptj/75.8.694
41. Bertrand-Charette M, Dambreville C, Bouyer LJ, Roy JS. Systematic review of motor control and somatosensation assessment tests for the ankle. BMJ Open Sport Exerc Med 2020;6(1):e000685. doi:10.1136/bmjsem-2019-000685
42. Gribble PA, Hertel J, Plisky P Using the star excursion balance test to assess dynamic posturalcontrol deficits and outcomes in lower extremity injury: a literature and systematic review. J Athl Train 2012;47(3):339-357 doi:10.4085/ 1062-6050-47.3.08
43. Myers JB, Riemann BL, Hwang JH, Fu FH, Lephart SM. Effect of peripheral afferent alteration of the lateral ankle ligaments on dynamic stability Am J Sports Med. 2003;31(4):498-506. doi:10.1177/ 03635465030310040401
44. Gokeler A, Welling W, Benjaminse A, Lemmink K, Seil R, Zaffagnini S. A critical analysis of limb symmetry indices of hop tests in athletes after anterior cruciate ligament reconstruction: A case control study. Orthop Traumatol Surg Res. 2017;103(6):947-951. doi:10.1016/j.otsr.2017.02.015
45. Caffrey C, Docherty CL, Schrader J, Klossner J. The ability of 4 single-limb hopping tests to detect functional performance deficits in individuals with functional ankle instability J Orthop Sports Phys Ther 2009;39(11):799-806. doi:10.2519/jospt.2009.3042
46. Best R, Brüggemann P, Petersen W, et al. Aktuelle und neue konzepte in der behandlung akuter außenbandverletzungen des sprunggelenkescurrent and new concepts in the treatment of lateral ligament sprains in ankle sistorsions. Deutsche Zeitschrift für Sportmedizin. 2011;62(3):57-62.
47. Bleakley CM, McDonough SM, MacAuley DC. Some conservative strategies are effective when added to controlled mobilisation with external support after acute ankle sprain: a systematic review. Aust J Physiother 2008;54(1):7-20. doi:10.1016/ s0004-9514(08)70061-8
48. van der Wees PJ, Lenssen AF, Hendriks EJ, Stomp DJ, Dekker J, de Bie RA. Effectiveness of exercise therapy and manual mobilisation in ankle sprain and functional instability: a systematic review. Aust J Physiother 2006;52(1):27-37 doi:10.1016/ s0004-9514(06)70059-9
49. Fox J, Docherty CL, Schrader J, Applegate T. Eccentric plantar-flexor torque deficits in participants with functional ankle instability J Athl Train 2008;43(1):51-54. doi:10.4085/1062-6050-43.1.51
50. Bohannon RW Manual muscle testing: does it meet the standards of an adequate screening test? Clin Rehabil. 2005;19(6):662-667. doi:10.1191/ 0269215505cr873oa
51. Gomes JLE, Soares AF, Bastiani CE, de Castro JV Anterolateral talar palpation: A complementary test for ankle instability Foot Ankle Surg 2018;24(6):486-489. doi:10.1016/j.fas.2017.05.006
52. Gribble PA. Evaluating and differentiating ankle instability. J Athl Train. 2019;54(6):617-627. doi:10.4085/1062-6050-484-17
53. Wiebking U, Pacha TO, Jagodzinski M. An accuracy evaluation of clinical, arthrometric, and stress-sonographic acute ankle instability examinations. Foot Ankle Surg 2015;21(1):42-48. doi:10.1016/j.fas.2014.09.006
54. Johnson MR, Stoneman PD Comparison of a lateral hop test versus a forward hop test for functional evaluation of lateral ankle sprains. J Foot Ankle Surg 2007;46(3):162-174. doi:10.1053/ j.jfas.2006.12.007
55. Rammelt S, Richter M, Walther M. Frische außenbandruptur am oberen sprunggelenk. Leitlinien Unfallchirurgie 2017;AWMF(012-022):22.
56. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518. doi:10.1177/ 036354659101900518
57 Davies WT, Myer GD, Read PJ. Is it time we better understood the tests we are using for return to sport decision making following ACL reconstruction? A critical review of the hop tests. Sports Med 2020;50(3):485-495. doi:10.1007/s40279-019-01221-7
58. Read P, Mc Auliffe S, Wilson MG, Myer GD. Better reporting standards are needed to enhance the quality of hop testing in the setting of ACL return to sport decisions: a narrative review Br J Sports Med 2021;55(1):23-29. doi:10.1136/bjsports-2019-101245
59. Kotsifaki A, Korakakis V, Graham-Smith P, Sideris V, Whiteley R. Vertical and horizontal hop performance: Contributions of the hip, knee, and ankle. Sports Health. 2021;13(2):128-135. doi:10.1177/1941738120976363
60. Kotsifaki A, Van Rossom S, Whiteley R, et al. Single leg vertical jump performance identifies knee function deficits at return to sport after ACL reconstruction in male athletes. Br J Sports Med 2022;56(9):490-498. doi:10.1136/ bjsports-2021-104692
61. Ross MD, Fontenot EG. Test-retest reliability of the standing heel-rise test. Sport Rehabil 2000;9:117-123. doi:10.1123/jsr.9.2.117
62. Sman AD, Hiller CE, Rae K, et al. Predictive factors for ankle syndesmosis injury in football players: a prospective study. J Sci Med Sport. 2014;17(6):586-590. doi:10.1016/j.jsams.2013.12.009
63. Lindner M, Kotschwar A, Zsoldos RR, Groesel M, Peham C. The jump shot - a biomechanical analysis focused on lateral ankle ligaments. J Biomech 2012;45(1):202-206. doi:10.1016/ j.jbiomech.2011.09.012
64. Haitz K, Shultz R, Hodgins M, Matheson GO Testretest and interrater reliability of the functional lower extremity evaluation. J Orthop Sports Phys Ther. 2014;44(12):947-954. doi:10.2519/jospt.2014.4809
65. Arundale A, Silvers H, Logerstedt D, Rojas J, Snyder-Mackler L. An interval kicking progression for return to soccer following lower extremity injury Int J Sports Phys Ther 2015;10(1):114-127
66. Lees A, Asai T, Andersen TB, Nunome H, Sterzing T. The biomechanics of kicking in soccer: a review. J Sports Sci 2010;28(8):805-817 doi:10.1080/ 02640414.2010.481305
67. Shinkai H, Nunome H, Isokawa M, Ikegami Y. Ball impact dynamics of instep soccer kicking. Med Sci Sports Exerc 2009;41(4):889-897 doi:10.1249/ MSS.0b013e31818e8044
68. Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med 2002;30(1):45-50. doi:10.1177/ 03635465020300012101
Download: https://ijspt.scholasticahq.com/article/120205-a-rehabilitation-algorithm-after-lateral-ankle-sprains-inprofessional-football-soccer-an-approach-based-on-clinical-practice-guidelines/attachment/ 232819.pdf?auth_token=S0zEEjWsI-PuJhmILB_5
Download: https://ijspt.scholasticahq.com/article/120205-a-rehabilitation-algorithm-after-lateral-ankle-sprains-inprofessional-football-soccer-an-approach-based-on-clinical-practice-guidelines/attachment/ 232817.pdf?auth_token=S0zEEjWsI-PuJhmILB_5
Download: https://ijspt.scholasticahq.com/article/120205-a-rehabilitation-algorithm-after-lateral-ankle-sprains-inprofessional-football-soccer-an-approach-based-on-clinical-practice-guidelines/attachment/ 232815.jpg?auth_token=S0zEEjWsI-PuJhmILB_5
Download: https://ijspt.scholasticahq.com/article/120205-a-rehabilitation-algorithm-after-lateral-ankle-sprains-inprofessional-football-soccer-an-approach-based-on-clinical-practice-guidelines/attachment/ 232816.jpg?auth_token=S0zEEjWsI-PuJhmILB_5
Download: https://ijspt.scholasticahq.com/article/120205-a-rehabilitation-algorithm-after-lateral-ankle-sprains-inprofessional-football-soccer-an-approach-based-on-clinical-practice-guidelines/attachment/ 232814.jpg?auth_token=S0zEEjWsI-PuJhmILB_5
Download: https://ijspt.scholasticahq.com/article/120205-a-rehabilitation-algorithm-after-lateral-ankle-sprains-inprofessional-football-soccer-an-approach-based-on-clinical-practice-guidelines/attachment/ 232818.pdf?auth_token=S0zEEjWsI-PuJhmILB_5
Download: https://ijspt.scholasticahq.com/article/120205-a-rehabilitation-algorithm-after-lateral-ankle-sprains-inprofessional-football-soccer-an-approach-based-on-clinical-practice-guidelines/attachment/ 234045.docx?auth_token=S0zEEjWsI-PuJhmILB_5

Conor Smith1a , Dustin R. Grooms2,3,4 , Helen Bradley1
1 Howard Head Sports Medicine, Vail Health, 2 Ohio Musculoskeletal and Neurological Institute, Ohio University, 3 Division of Athletic Training, School of Applied Health Sciences and Wellness, College of Health Sciences and Professions, Ohio University, 4 Division of Physical Therapy, School of Rehabilitation and Communication Sciences, College of Health Sciences and Professions, Ohio University
Keywords: ACL, Alpine Skiing, Return to Sport, perceptual-motor-cognitive rehab https://doi.org/10.26603/001c.120285
Alpine skiing poses significant risks for anterior cruciate ligament (ACL) injury at both recreational and professional levels, which is compounded by high rates of re-injury
Despite the existence of return to sport (RTS) and return to snow protocols, the frequency of ACL re-injury has not been mitigated, raising doubts about protocol effectiveness. Current RTS protocols primarily focus on biomechanical and neuromuscular factors in isolation, neglecting the important perceptual-motor-cognitive changes associated with ACL injuries and the high cognitive demands of skiing. The purpose of this clinical commentary is to address the perceptual-motor-cognitive demands specific to alpine skiing, evaluate RTS testing for skiers, and propose updated standards for testing and return to snow progressions that incorporate these considerations.
5
Participation in alpine skiing, whether recreationally or professionally, has a high risk of injury The injury rate for recreational skiers was approximately 0.5-1.98 per 1000 skier days, or approximately one injury per 10,000 lifts rides.1‑5 Knee injury accounts for 27-41% of all injuries at ski resorts, with an anterior cruciate ligament (ACL) and medial cruciate ligament (MCL) sprain being the most common diagnoses.6‑11 In professional skiing athletes the injury rate is even higher, at 36.2-36.7 injuries per 100 athletes, with the most common injured body part also being the knee at 35.6%, consistent with recreational skiing statistics.12 One-third of injuries were considered severe, resulting in a loss of training and competition for a minimum of 28 days or more.1,12
Specifically among skiers, 19-46.7% of competitive alpine skiers suffer a re-injury to their reconstructed ACL or contralateral ACL, which is consistent or more frequent than the general athletic population.13‑16 Multiple studies describe functional asymmetries and deficits that persist after return to play (RTP) in ACL reconstructed athletes
that could possibly explain this high injury risk.13,17‑20 Despite return to sport (RTS) and return to snow protocols attempting to provide quantitative testing to ensure sport readiness and reduce reinjury risk, the rate of ACL re-injury remains high. RTS protocols are primarily based on biomechanical and neuromuscular function including range of motion, strength, and functional tests such as jumping and hopping and sport specific testing.21 Return to snow protocols for high level skiers include similar multifactorial measures, but also suggest supervised progression of “ onsnow” drills to return to full function and racing performance.22,23
An aspect of ACL injury and recovery that has unique implications for the high-speed perceptual-motor-cognitive demands of skiing is the neuroplastic adaptations (altered brain and neural activity) recently described to be associated with the injury Perceptual-motor-cognitive is an integrated demand that includes maintaining dynamic joint stability in a highly variable perceptual environment while engaged in very rapid cognitiveplan updates with distractors. Typically, rehabilitation and RTS testing after ACL reconstruction (ACLR) focuses on restoring functional abil-
Corresponding Author: Conor Smith, PT, DPT 1425 Bighorn Rd, Helena, MT, 59602
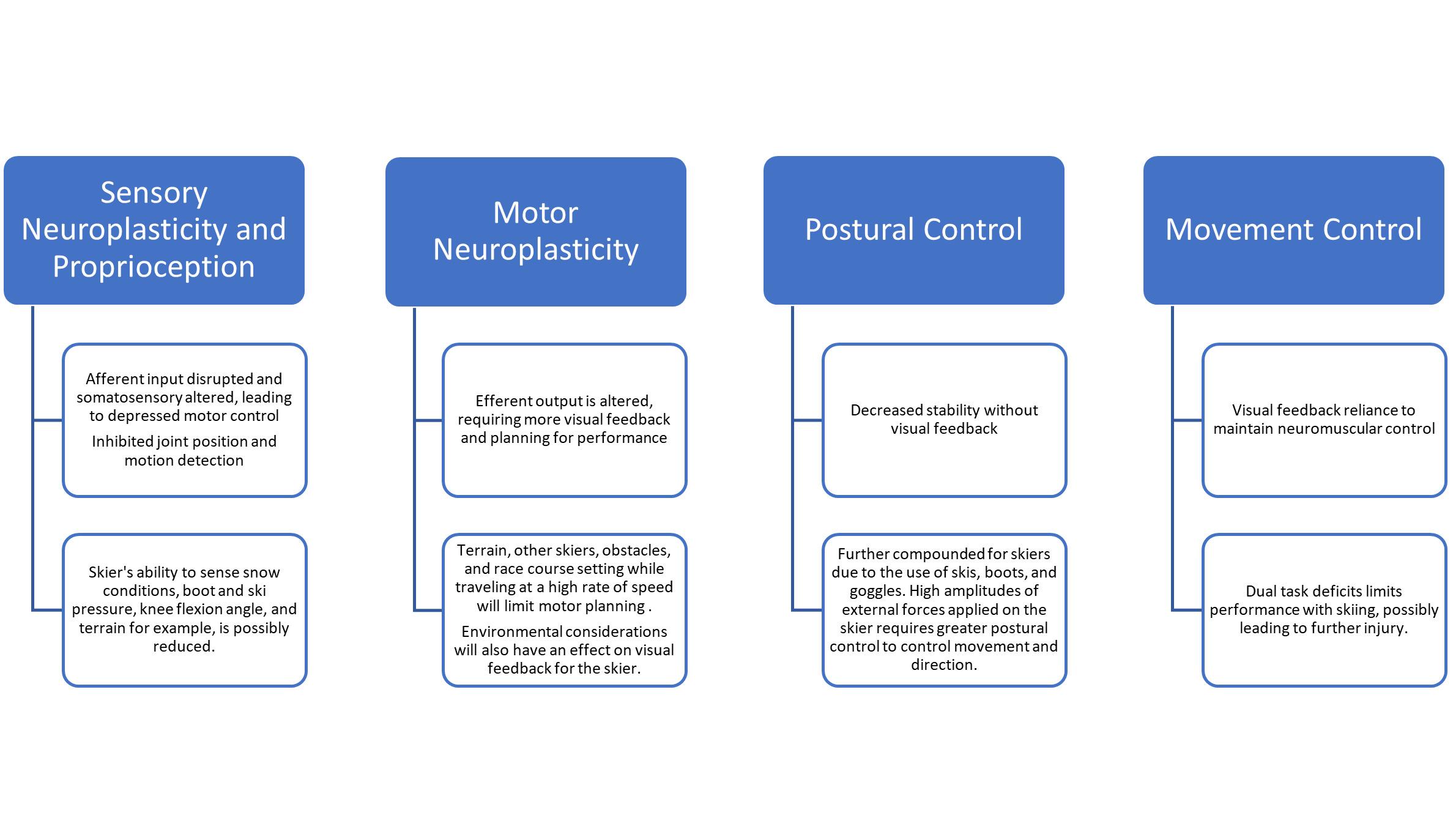
Figure 1. The conceptual framework for neurologic and visual-motor adaptations after ACL injury, and the perceptual-motor-cognitive neuroplastic adaptations that possibly occur within alpine skiing.
Adapted from Grooms et al 2015.24
ity (addressing interlimb asymmetry in muscle power, rate of force development, maximal strength) and psychological readiness.21‑23 However, injury deafferentation and associated pain, muscle atrophy, and movement compensations contribute to neuroplastic adaptations and variations in motor control upon return to sport.24 Specifically, it appears that the injury associated neuroplastic alterations result in changes in perceptual-motor-cognitive neural activity that may explain the elevated dual-ask cost and injury risk.
The loss of movement quality anddual-task cost for those with ACLR is theorized to be secondary to the injury disruption in sensory processing resulting in increased utilization of visual input andvisual-cognitive processing capacity to compensate to maintain dynamic joint stability 25‑37 However, skiing requires sensory processing in a rapidly changing external environment that can overwhelm the developed neural compensations and may contribute to a reduced ability to maintain coordination and increase contralateral ACL injury or re-injury risk.38‑41 Thus, to better prepare athletes and ensure adequate sport readiness with RTS testing clinicians may consider creating different challenges to sensory andcognitive processing that have implications for maintaining neuromuscular control in sport. Currently, there is a gap in knowledge regarding how best to quantify perceptual-motor-cognitive demands in sport, especially in alpine skiing, to inform the safe return to snow after an ACL injury The purpose of this clinical commentary is to address the perceptual-motor-cognitive demands specific to alpine skiing, evaluate RTS testing for skiers, and propose updated standards for testing and re-
turn to snow progressions that incorporate these considerations.
For alpine skiers specifically, there are a variety of environmental and equipment considerations that contribute to external perturbations and cognitive load. Environmentally, colder weather affects fine motor control, postural control, balance, and proprioception likely attributed to a decrease in muscle contraction velocity and nerve conduction velocity 42‑44 Variable wind and snow conditions can reduce visibility and create unfavorable changes to the course surface; however, there is no agreement among coaches and researchers about which snow conditions, snow-covered or water-injected, are safest for alpine skiers.45 Differing snow conditions within a course can bring athletes close to their physical and technical limits, possibly increasing risk for injury 45 Changes in course conditions from those at which the athlete practiced and trained would influence their sensory predictions and may slow postural corrections, increasing risk of injury at high competition speeds. Course conditions and neuroplasticity of injury could limit overall performance if perceptual and cognitive processing speed is not at a level at which the skier is accustomed to.
Skis are also an equipment-related ACL injury risk factor due to differences in ski length, tip width of the ski, standing height at the rear ski binding component, and in standing height ratio (percentage between front and rear component heights and how it relates to the angle of the boot sole when inserted into the binding).46 A ski bindings DIN (Deutsches Institut für Normung, German Institute for Standardization) setting or release force of the ski is equally important for preventing injury, failure of binding release at the moment of accident, the ski acts as a lever to bend or twist the leg, leading to a potential severe knee injury 47 Failure of binding release during a fall resulting in an ACL rupture has been often reported in falls (78%) and is significantly more often with females compared to men.48 Although ski and boot technology has improved in recent years in attempts to enhance skier safety, overall, the skibinding interface, bindings, and boots impair proprioception from the lower extremity, thus increasing the risk for falls and mistakes, and cause an increased strain on the ACL at higher loads.49‑51 Helmets and ski googles have also been shown to limit visual performance, which can reduce reaction time to peripheral stimuli or limit visual input for postural control, thus putting a skier at risk for injury.52,53
The unique high speeds of alpine skiing places additional cognitive and motor planning stress that can be influenced by subtle alterations in course terrain. Course structures such as rolling and dipping terrain transitions, placement and number of gates during high level racing, jumps, and speed of the terrain/slope reduces the time that skiers have to anticipate and adapt to technically demanding sections, increasing the risk for mistakes.54 For the recreational skier, while variable skiing environments are comparable with alpine ski racers, there is also the added perceptualmotor-cognitive load of other skiers sharing the same terrain. This could have a negative impact on skiing performance through impaired sensorimotor prediction due to added unanticipated reactions and rapid fluctuations in distracted attention from skiing, while maintaining neuromuscular control and efficient visual processing.
Athletes following ACLR have been theorized to have lost sensory integration efficiency, requiring increased neural activity to perform a motor task compared to a control group.30 The amount of afferent information from external perturbation that a skier must process is accompanied by the intensive motor requirements of downhill skilling with increased muscle force and coordination timing demands. The need for high level technical skills and sensory processing has been shown to improve the ability to anticipate a collision or fall, reducing the impact severity for athletes.55 The combination of ACLR associated neuroplastic alterations and skiing perceptual-cognitive demands may contribute to the high rates of re-injury in this population, despite RTS testing suggesting that functional recovery has occurred. This points to the need to consider aspects of sport requirements and injury adaptations not
typically captured in RTS testing. To that end the authors suggest that perceptual-motor-cognitive additions could be a missing element and suggest methods to accommodate it in the return to snow progression.
Despite accumulating evidence that ACL injury induces compensatory neuroplastic adaptations within neural circuits, current return-to-snow testing does not adequately capture the ability to effectively respond to all aspects of the intense perceptual-motor-cognitive demands that can be encountered when skiing. Current RTS decision-making is primarily based on maximal muscle strength testing and closed motor skills, reaching a specific milestone that nears limb symmetry or allometric scaling with normative data.21,56 Multiple studies report a battery of objective neuromuscular or psychological readiness testing that may include variable challenges to simulate sport related movements, however, comprehensive assessment of perceptual, motor, and cognitive capabilities is often lacking within RTS testing.21‑23,57 In the clinical setting, it is difficult to simulate the complex sport environment of skiing due to the inability to simulate the forward inertia of skiers via the downhill landscape, requirement of equipment, and snow variations. However, it is possible to challenge the perceptual-motor-cognitive demands of skiers during testing by integrating perceptual-motor-cognitive elements into the established RTS tests within this population. As highlighted previously, this appears to be an element that could be missing from testing and return to snow progressions and is essential for alpine skiers. Common in athletics including alpine skiers, additional functional movement and strength tests performed in RTS testing focus on the single leg hop, single leg crossover hop, and the single leg jump/ strength testing on force plates.21,22,58,59 Without visualcognitive stress to simulate the sporting environment, athletes could possibly compensate and give the appearance of restored function, and if released to sport early, can possibly still be at high risk for re-injury The authors suggest that a full skiing environmental simulation is not required, but more simply challenging of the underlying physiology required by the sport. Just as strength training improves capacity for muscular performance, so to can coordination training under perceptual-cognitive demands improves capacity to maintain dynamic joint stability upon returning to the snow environment. In this way the authors challenge the elements of rapid decision making and motor plan updating, attention on a changing environment and cognition directed away from knee kinematics as key constructs to train the underlying physiology to provide the capacity transfer to skiing.38
While standard functional testing allows for ease of set up and replication, and valuable baseline data about limb
asymmetries post ACLR; standardized tests lack perceptual-motor-cognitive elements that could better simulate sport demand for the alpine skier RTS testing with perceptual, motor, and cognitive features could be utilized to fully understand the dual task deficit that occurs after an ACL injury. Researchers are developing reliable and replicable tests with perceptual and cognitive stress modified from common functional RTS challenges.
Hop tests are a common measure to determine if an athlete is fit to return to sport but are often performed without the dual task challenged needed to replicate the demands of skiing. Recent research has been focused on improving generic hop tests, by adding elements of perceptual-cognitive elements of reaction time, visual tracking, spatial awareness, and visual working memory along with motor performance.60‑62 A jump test that may have greater applications to the stability required for skiing could be the visual-cognitive side hop test.63 Skiers must overcome the dynamic balance of forces from gravity and vertical/horizontal ground reaction forces as well as utilize motor control to make turns possible. The visual-cognitive side hop test could possibly simulate the lateral ground reaction force that is exerted on the skier during a turn while providing a dual task similar to an alpine event.63 It is also hypothesized that side hop tests can reveal greater asymmetries in athletes with prior ACL injuries as well as elicit greater knee valgus, hip adduction, and medial rotation.64 These angles are often stressed in the various phases of a ski turn suggesting that this test could be effective in assessing return to snow capabilities.65 Further jump-landings can be incorporated via a double leg take off with single leg landing with an unanticipated visuomotor stimuli indicating which leg to land on (left or right).66,67
Similar perceptual-cognitive additions can be integrated into already commonly done strength and jump assessments in order to assess dual-task cost on rate of force development, reaction time, and peak force production. Simple computer programming, interactive LED training lights or even verbal/visual commands from a practitioner can be utilized to complement strength testing. For instance, an isometric closed chain single leg press at 60 degrees (meet resistance) or an 80/20 jump test on force plates can be tested with stop/go lights or screen programming. Strength, reaction time or force development can be compared to the uninvolved limb to see if a 90% limb symmetry index can be maintained under dual task challenged conditions (as will occur in sport/skiing).
To determine reaction time, testing can be analyzed with applications and slow-motion video technology, which also enables the practitioner to analyze movement patterns. Movement patterns can also be evaluated in real-time with the landing error scoring system (LESS) or balance error scoring system (BESS), providing a movement quality out-
come to determine dual-task cost. Chabaan and colleagues developed a creative instrument in order to create both motor and cognitive tasks during functional testing (drop jumps, single limb jump tests, cutting) that can be easily applied and measured based on low or high tech resources within clinic.68 A calculation for dual task cost was also proposed during specific motor tasks in order to quantify the motor performance and movement quality with or without cognitive load, in order to compare healthy and surgical limb. This concept of a dual-task cost application can also be provided to snow specific settings.
Replicating an alpine environment in clinic is difficult to adequately stress the skier for safe return to sport. Utilization of helmets and goggles during return to snow testing can limit visual field and proprioception, and more accurately reproduce challenges specifically for athletes trying to return to alpine skiing. For example, helmets and goggles can be applied to hop testing, balance training, lateral agility, and closed chain strength training. Furthermore, performance before and after equipment application can be measured while assessing limb symmetry (strength, reaction time, and distance), and quality of motion. The added visual perturbations and perceptual-cognitive load with equipment changes could be an effective tool to bridge inclinic return to RTS testing with return to snow training and possibly reduce sensorimotor prediction errors resulting in poor performance. Perceptual-cognitive challenges should continue to be applied for alpine skiers, both recreational and professional, when cleared to return to snow
Progression-based return to snow is may ultimately prove to be highly beneficial for skiers returning from an ACL injury. Kokmeyer and associates suggest a return to snow program that is progression-based with differing intensities, focus, and durations as well as example drills that can be utilized while on snow 23 They recommend that after a skier completes their clinical functional sports test, they start a return to snow program over the course of 8+ weeks, typically during their 6-9 months post operative timeline.23 Although directed primarily at elite alpine skiers, a similar strategy can be utilized towards the recreational skier, under the supervision and guidance of a medical provider. The return to snow program described by Kokmeyer and colleagues included a variety of drills that challenged proprioception but could be further modified to progress perceptual-motor-cognitive load while on snow.23 Perceptual load was challenged by adding various challenges and drills on a single leg when turning or sliding. Additional challenge during single leg drills can be progressed by varying ski lengths and boot stiffness. Although an effective return to snow progression, there is nothing specific on visual perturbations, environmental considerations, and ways that
multiple challenges can be combined to adequately perceptually, cognitively, and motor stress these athletes. Adding perceptual-cognitive challenge to these drills can be achieved by reducing visual input, adding reaction time or anticipatory elements, and varying environmental constraints (Tables 1 and 2). Exercise progressions on snow can also be altered based on the level of skier in order to adequately test and assess their ability to perform an activity with cognitive stress (Tables 1 and 2). Recreational skier progressions may involve stressors more focused on visual and environmental elements whereas professional skiers could focus more on visual perturbations, varying proprioceptive and perceptual inputs (turns/equipment/course terrain), and reactionary elements similar to that in the racing environment. Perceptual-motor-cognitive stressors should not be progressed by adding multiple elements in a singular session, but to implement one element at a time and evaluate responses before progressing. For example, introducing night skiing (visual challenge) with a new proprioceptive challenge (such as a different ski length than what they usually ski with) may produce too much of a challenge for the athlete resulting in reduced performance. Although difficult to objectively ensure return to competition readiness, especially with alpine skiers and on-snow progressions, drills designed to stress perceptual-motor-cognitive elements would help simulate an environment and cognitive load that would possibly give a skier the psychological and cognitive capacity to return to prior level of function. As opposed to depending on physical recovery and hoping that performance is not degraded when under the cognitive demand of sport.
It is important to note that the return to snow progression doesn’t look at a specific moment in time, but throughout the entire continuum of rehabilitation in order to return to snow In order to safely and effectively return to snow at a high-performance level, whether that is recreationally or professionally, consistent quantitative and qualitative testing is required at multiple timelines during the rehabilitative program. Passing objective criteria in clinic allows the athlete to return to participation of on snow training activities. It is stressed that until guidelines are established for return to participation with dual-task testing, caution is urged for athletes that have large dual task costs with traditional testing battery under perceptualcognitive-motor load.68 The athlete must also safely perform all training progressions with added perceptual-cognitive-motor elements, if possible, under the supervision of coaching and medical professionals in order to return to snow Quantification of readiness for a professional athlete would be prior level of performance (no more than a 10% degradation in dual task cost) during time trials in a simulated environment comparable to that of a professional event with appropriate motor control. Monitoring of all elements of the return to snow continuum allows practitioners to make informed decisions and increase the likelihood that the athlete returns to skiing at a high level of performance.69
In conclusion, alpine skiing is a high-risk sport for both the recreational and high-level athlete, with a high rate of re-injury seen in athletes that have suffered an initial ACL injury and reconstruction. This can be attributed to several unique factors included in the sport of alpine skiing, including equipment, positions that place extreme forces and torques on the knee, and an extraordinary demand for neuromuscular control, endurance, strength, and eccentric control in the lower extremity.23 Alpine skiing is an openskilled sport, where skiers are exposed to multiple stimuli in which athletes must make decisions in an unpredictable, dynamically changing environment.38 Alpine skiers who suffer from an ACL injury, despite reconstructive surgery, demonstrate perceptual, cognitive, and motor processes that possibly induce a neuroplastic change within the athlete thus increasing the risk of re-injury. These deficits are routinely trained within a clinical setting in order to return an athlete to their sport at a high level; however, a patient’s ability to perform under dual task stress is typically not measured due to the difficulty of objectifying cognition and performance. Strategies have now been introduced to assist in the assessment of an alpine skier’s return to snow functional testing in conjunction with neurocognitive and physical performance. Furthermore, perceptual-motor-cognitive challenges can be added to an already established return to snow program to improve safety in a skier’s progression to prior function and bridge the gap between clinic and on-snow rehabilitation.
Dustin Grooms reports the following grants and potential conflicts of interest:
US Department of Department of Defense Congressionally Directed Medical Research Program Peer Reviewed Orthopaedic Research Program (research award W81XWH-18-1-0707); National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR076153, R01AR077248) and Consulting Fees from Kinesport physical therapy (France).
The authors would like to express deepest appreciation to Howard Head Sports Medicine and the Howard Head Sports Residency Program for the support of this project. Special thanks to Dane Ringquist DPT and Delaney Smith for photography, as well as Brooke Milliet DPT for her assistance with skiing specific perceptual-motor-cognitive task development.
Submitted: October 04, 2023 CDT, Accepted: June 12, 2024 CDT
© The Author(s)
Table 1. Return to snow with examples of perceptual-cognitive-motor progressions for the recreational skier
Perceptual-Motor-Cognitive Challenge
Challenge Progression
Easy
Moderate
Hard
Advanced
In-Clinic Options
Visual
Sunny/Night skiing
Dark or light tint goggles
With/without goggles
Skiing with visual tracking/reading (reading trail signs for example)
Reduced visual field goggles
Proprioceptive
Wedge vs hockey stop
Sliding vs Carving
Varying Muscle Fatigue
Ski length, width, type, skiing with/ without pole
Varying boot stiffness
Eyes closed or Head turns
LED training lights
Visual cue cards
Eyes closed
Stroboscopic glasses
Inside edge skiing
Double or single leg balance on ½ foam roller or BOSU ball
Reaction time/ anticipatory Environmental Changes
Distracted skiing (music, mental tasks, etc)
Randomized stop/starts
Speed variability
Groomed runs
Wet/sticky snow
Powder days
Randomized turns Foggy/low light
Variable slopes
Skiing in trees/ moguls
Unanticipated Perturbations from PT or exercise ball
Virtual reality
LED training lights
Visual cue cards
Moguls
Large gym with distractions
Loud music
Varying surfaces (turf, grass, track, concrete, etc)
Different aspects of perceptual-cognitive-motor challenge should not be progressed all at one time, for example, skiing at night on a mogul run. Challenges should be progressed one at a time in order to allow safe and achievable goals for the recreational skier
Table 2. Return to snow with examples of perceptual-cognitive-motor progressions for the professional skier
Perceptual-Motor-Cognitive Challenge
Challenge Progression
Easy
Visual
Skiing with gaze stabilization
Moderate Number of gates and distance between gates
Skiing with head turns for vestibular training
Hard Reduced visual field goggles
Stroboscopic glasses
Advanced
In-Clinic Options
Proprioceptive
Double leg slide slipping, diagonal, sliding turns.
Single leg sliding turns with heel lift or cross hip opposite ski (Javelin Turn)
Varying boot stiffness and ski length/width
Turns with 1000 ft stepping
Eyes closed Turns with one leg lifted
LED training lights
Visual cue cards
Eyes closed
Stroboscopic glasses
Double or single leg balance on ½ foam roller or BOSU ball
Reaction time/ anticipatory
Coach led randomized stops and starts
Coach led randomized turns
Stubby/brush track
Environmental Changes
Groomed runs
Cold vs Hot Temperatures
Variable Slopes
Multiple practice courses on slope
Variable slalom course
Vary racing events: downhill, super G, giant slalom, slalom
Unanticipated Perturbations from PT or exercise ball
Virtual reality
LED training lights
Visual cue cards
Snow coverage course
Foggy/flat light
Injected Course
Large gym with distractions
Loud music
Varying surfaces (turf, grass, track, concrete, etc)


Figure 3. Javelin turns or opposite ski crossed hip opposite ski turns to increase perceptual stress on outside ski during turn as well as postural stress under load.

Figure 4. Single leg turns (same leg turning both directions) for perceptual and postural stress on a single leg during different angles and torque of a turn.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Stenroos AJ, Handolin LE. Alpine skiing injuries in Finland – a two-year retrospective study based on a questionnaire among Ski racers. BMC Sports Science, Medicine and Rehabilitation 2014;6(1):9. doi:10.1186/ 2052-1847-6-9
2. Ekeland A, Rødven A. Skiing and boarding injuries on Norwegian slopes during two winter seasons. Journal of ASTM International (JAI) 2010;7(4). doi:10.1520/JAI102817
3. Castellani C, Singer G, Eibisberger M, et al. An epidemiologic analysis of winter sport accidents on ski slopes comparing two seasons. J Sports Med Phys Fitness 2019;59(4):648-654. doi:10.23736/ s0022-4707.18.08665-6
4. Ruedl G, Philippe M, Sommersacher R, Dünnwald T, Kopp M, Burtscher M. [Current incidence of accidents on Austrian ski slopes]. Sportverletz Sportschaden. 2014;28(4):183-187. doi:10.1055/ s-0034-1385244
5. Chen N, Yang Y, Jiang Y, Ao Y Injury patterns in a large-scale ski resort in the host city of 2022 Winter Olympic Games: a retrospective cross-sectional study BMJ Open 2020;10(11):e037834. doi:10.1136/ bmjopen-2020-037834
6. Davey A, Endres NK, Johnson RJ, Shealy JE. Alpine Skiing Injuries. Sports Health 2019;11(1):18-26. doi:10.1177/1941738118813051
7. Coury T, Napoli AM, Wilson M, Daniels J, Murray R, Milzman D Injury patterns in recreational alpine skiing and snowboarding at a mountainside clinic. Wilderness Environ Med. 2013;24(4):417-421. doi:10.1016/j.wem.2013.07.002
8. Patrick E, Cooper JG, Daniels J. Changes in Skiing and Snowboarding Injury Epidemiology and Attitudes to Safety in Big Sky, Montana, USA: A Comparison of 2 Cross-sectional Studies in 1996 and 2013. Orthop J Sports Med. 2015;3(6):2325967115588280. doi:10.1177/2325967115588280
9. Sulheim S, Holme I, Rødven A, Ekeland A, Bahr R. Risk factors for injuries in alpine skiing, telemark skiing and snowboarding--case-control study Br J Sports Med 2011;45(16):1303-1309. doi:10.1136/ bjsports-2011-090407
10. Ruedl G, Kopp M, Sommersacher R, Woldrich T, Burtscher M. Factors associated with injuries occurred on slope intersections and in snow parks compared to on-slope injuries. Accid Anal Prev 2013;50:1221-1225. doi:10.1016/j.aap.2012.09.019
11. Burtscher M, Gatterer H, Flatz M, et al. Effects of modern ski equipment on the overall injury rate and the pattern of injury location in Alpine skiing. Clin J Sport Med 2008;18(4):355-357 doi:10.1097/ MJT.0b013e31815fd0fe
12. Florenes TW, Bere T, Nordsletten L, Heir S, Bahr R. Injuries among male and female World Cup alpine skiers. Br J Sports Med 2009;43(13):973-978. doi:10.1136/bjsm.2009.068759
13. Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD Risk of Secondary Injury in Younger Athletes After Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2016;44(7):1861-1876. doi:10.1177/ 0363546515621554
14. Pujol N, Rousseaux Blanchi MP, Chambat P The Incidence of Anterior Cruciate Ligament Injuries among Competitive Alpine Skiers: A 25-year Investigation. Am J Sports Med 2007;35(7):1070-1074. doi:10.1177/ 0363546507301083
15. Stevenson H, Webster J, Johnson R, Beynnon B. Gender differences in knee injury epidemiology among competitive alpine ski racers. Iowa Orthop J. 1998;18:64-66.
16. Csapo R, Runer A, Hoser C, Fink C. Contralateral ACL tears strongly contribute to high rates of secondary ACL injuries in professional ski racers. Knee Surg Sports Traumatol Arthrosc 2021;29(6):1805-1812. doi:10.1007/ s00167-020-06234-8
17 Lepley LK. Deficits in Quadriceps Strength and Patient-Oriented Outcomes at Return to Activity After ACL Reconstruction: A Review of the Current Literature. Sports Health 2015;7(3):231-238. doi:10.1177/1941738115578112
18. Mattacola CG, Perrin DH, Gansneder BM, Gieck JH, Saliba EN, McCue FC. Strength, Functional Outcome, and Postural Stability After Anterior Cruciate Ligament Reconstruction. J Athl Train. 2002;37(3):262-268.
19. Myer GD, Ford KR, Brent JL, Hewett TE. An Integrated Approach to Change the Outcome Part II: Targeted Neuromuscular Training Techniques to Reduce Identified ACL Injury Risk Factors. J Strength Cond Res. 2012;26(8):2272-2292. doi:10.1519/ JSC.0b013e31825c2c7d
20. Paterno MV, Ford KR, Myer GD, Heyl R, Hewett TE. Limb Asymmetries in Landing and Jumping 2 Years Following Anterior Cruciate Ligament Reconstruction. Clin J Sport Med. 2007;17(4):258. doi:10.1097/JSM.0b013e31804c77ea
21. Davies GJ, McCarty E, Provencher M, Manske RC. ACL Return to Sport Guidelines and Criteria. Curr Rev Musculoskelet Med. 2017;10(3):307-314. doi:10.1007/ s12178-017-9420-9
22. Jordan MJ, Morris N, Lane M, et al. Monitoring the Return to Sport Transition After ACL Injury: An Alpine Ski Racing Case Study Front Sports Act Living 2020;2. doi:10.3389/fspor.2020.00012
23. Kokmeyer D, Wahoff M, Mymern M. Suggestions from the field for return-to-sport rehabilitation following anterior cruciate ligament reconstruction: alpine skiing. J Orthop Sports Phys Ther. 2012;42(4):313-325. doi:10.2519/jospt.2012.4024
24. Grooms D, Appelbaum G, Onate J. Neuroplasticity Following Anterior Cruciate Ligament Injury: A Framework for Visual-Motor Training Approaches in Rehabilitation. J Orthop Sports Phys Ther 2015;45(5):381-393. doi:10.2519/jospt.2015.5549
25. Criss CR, Melton MS, Ulloa SA, et al. Rupture, reconstruction, and rehabilitation: A multidisciplinary review of mechanisms for central nervous system adaptations following anterior cruciate ligament injury The Knee 2021;30:78-89. doi:10.1016/j.knee.2021.03.009
26. Young SW, Valladares RD, Loi F, Dragoo JL. Mechanoreceptor Reinnervation of Autografts Versus Allografts After Anterior Cruciate Ligament Reconstruction. Orthop J Sports Med 2016;4(10):2325967116668782. doi:10.1177/ 2325967116668782
27 Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthopaedica Scandinavica 2002;73(3):330-334. doi:10.1080/ 000164702320155356
28. Baumeister J, Reinecke K, Liesen H, Weiss M. Cortical activity of skilled performance in a complex sports related motor task. Eur J Appl Physiol. 2008;104(4):625-631. doi:10.1007/s00421-008-0811-x
29. Baumeister J, Reinecke K, Schubert M, Weiß M. Altered electrocortical brain activity after ACL reconstruction during force control. J Orthop Research 2011;29(9):1383-1389. doi:10.1002/ jor.21380
30. Chaput M, Onate JA, Simon JE, et al. Visual cognition associated with knee proprioception, time to stability, and sensory integration neural activity after ACL reconstruction. J Orthop Res. 2022;40(1):95-104. doi:10.1002/jor.25014
31. Ahmadi P, Salehi R, Mehravar M, Goharpey S, Negahban H. Comparing the effects of external focus of attention and continuous cognitive task on postural control in anterior cruciate ligament reconstructed athletes. Neuroscience Letters. 2020;715:134666. doi:10.1016/j.neulet.2019.134666
32. Lion A, Gette P, Meyer C, Seil R, Theisen D Effect of cognitive challenge on the postural control of patients with ACL reconstruction under visual and surface perturbations. Gait & Posture 2018;60:251-257. doi:10.1016/j.gaitpost.2017.12.013
33. Mohammadi-Rad S, Salavati M, EbrahimiTakamjani I, et al. Dual-Tasking Effects on Dynamic Postural Stability in Athletes With and Without Anterior Cruciate Ligament Reconstruction. J Sport Rehabil 2016;25(4):324-329. doi:10.1123/ jsr.2015-0012
34. Negahban H, Ahmadi P, Salehi R, Mehravar M, Goharpey S. Attentional demands of postural control during single leg stance in patients with anterior cruciate ligament reconstruction. Neuroscience Letters 2013;556:118-123. doi:10.1016/ j.neulet.2013.10.022
35. Stone AE, Roper JA, Herman DC, Hass CJ. Cognitive Performance and Locomotor Adaptation in Persons With Anterior Cruciate Ligament Reconstruction. Neurorehabil Neural Repair. 2018;32(6-7):568-577 doi:10.1177/ 1545968318776372
36. Swanik CB, Covassin T, Stearne DJ, Schatz P. The Relationship between Neurocognitive Function and Noncontact Anterior Cruciate Ligament Injuries. Am J Sports Med. 2007;35(6):943-948. doi:10.1177/ 0363546507299532
37 Houck JR, Wilding GE, Gupta R, De Haven KE, Maloney M. Analysis of EMG patterns of control subjects and subjects with ACL deficiency during an unanticipated walking cut task. Gait & Posture 2007;25(4):628-638. doi:10.1016/ j.gaitpost.2006.07.001
38. Piskin D, Benjaminse A, Dimitrakis P, Gokeler A. Neurocognitive and Neurophysiological Functions Related to ACL Injury: A Framework for Neurocognitive Approaches in Rehabilitation and Return-to-Sports Tests. Sports Health. 2022;14(4):549-555. doi:10.1177/19417381211029265
39. Kakavas G, Malliaropoulos N, Bikos G, et al. Periodization in Anterior Cruciate Ligament Rehabilitation: A Novel Framework. Medical Principles and Practice. 2020;30(2):101-108. doi:10.1159/000511228
40. Bere T, Flørenes TW, Krosshaug T, Nordsletten L, Bahr R. Events leading to anterior cruciate ligament injury in World Cup Alpine Skiing: a systematic video analysis of 20 cases. Br J Sports Med 2011;45(16):1294-1302. doi:10.1136/ bjsports-2011-090517
41. Swanik C “Buz.” Brains and Sprains: The Brain’s Role in Noncontact Anterior Cruciate Ligament Injuries. J Athl Train. 2015;50(10):1100-1102. doi:10.4085/1062-6050-50.10.08
42. Racinais S, Oksa J. Temperature and neuromuscular function. Scandinavian Journal of Medicine & Science in Sports 2010;20(s3):1-18. doi:10.1111/j.1600-0838.2010.01204.x
43. Racinais S, Gaoua N, Mtibaa K, Whiteley R, Hautier C, Alhammoud M. Effect of Cold on Proprioception and Cognitive Function in Elite Alpine Skiers. Int J Sports Phys Perf. 2017;12(1):69-74. doi:10.1123/ijspp.2016-0002
44. Mäkinen TM, Rintamäki H, Korpelainen JT, et al. Postural Sway During Single and Repeated Cold Exposures. Aviation, Space, and Environmental Medicine 2005;76(10):947-953.
45. Spörri J, Kröll J, Gilgien M, Müller E. How to Prevent Injuries in Alpine Ski Racing: What Do We Know and Where Do We Go from Here? Sports Med 2017;47(4):599-614. doi:10.1007/s40279-016-0601-2
46. Ruedl G, Posch M, Tecklenburg K, et al. Impact of ski geometry data and standing height ratio on the ACL injury risk and its use for prevention in recreational skiers. Br J Sports Med 2022;56(19):1104-1109. doi:10.1136/ bjsports-2021-105221
47. Natri A, Beynnon BD, Ettlinger CF, Johnson RJ, Shealy JE. Alpine Ski Bindings and Injuries. Sports Med. 1999;28(1):35-48. doi:10.2165/ 00007256-199928010-00004
48. Ruedl G, Helle K, Tecklenburg K, Schranz A, Fink C, Burtscher M. Factors associated with self-reported failure of binding release among ACL injured male and female recreational skiers: a catalyst to change ISO binding standards? Br J Sports Med. 2016;50(1):37-40. doi:10.1136/bjsports-2015-095482
49. Eberle R, Heinrich D, Kaps P, Oberguggenberger M, Nachbauer W. Effect of ski boot rear stiffness (SBRS) on maximal ACL force during injury prone landing movements in alpine ski racing: A study with a musculoskeletal simulation model. J Sports Sci 2017;35(12):1125-1133. doi:10.1080/ 02640414.2016.1211309
50. Senner V, Michel F, Lehner S. Ski EquipmentRelated Measures to Reduce Knee Injuries – Review of the Potential for Further Technical Improvements in Recreational Alpine Skiing. Published online 2013.
51. Jordan MJ, Aagaard P, Herzog W Anterior cruciate ligament injury/reinjury in alpine ski racing: a narrative review. Open Access Journal of Sports Medicine 2017;8:71-83. doi:10.2147/OAJSM.S106699
52. Očić M, Bon I, Ružić L, Cigrovski V, Rupčić T The Influence of Protective Headgear on the Visual Field of Recreational-Level Skiers. International Journal of Environmental Research and Public Health 2022;19(17):10626. doi:10.3390/ijerph191710626
53. Ruedl G, Herzog S, Schöpf S, et al. Do Ski Helmets Affect Reaction Time to Peripheral Stimuli? Wilderness & Environmental Medicine. 2011;22(2):148-150. doi:10.1016/j.wem.2010.12.010
54. Gilgien M, Spörri J, Kröll J, Crivelli P, Müller E. Mechanics of turning and jumping and skier speed are associated with injury risk in men’s World Cup alpine skiing: a comparison between the competition disciplines. Br J Sports Med. 2014;48(9):742-747. doi:10.1136/bjsports-2013-092994
55. Mihalik JP, Blackburn JT, Greenwald RM, Cantu RC, Marshall SW, Guskiewicz KM. Collision Type and Player Anticipation Affect Head Impact Severity Among Youth Ice Hockey Players. Pediatrics 2010;125(6):e1394-e1401. doi:10.1542/ peds.2009-2849
56. Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current Concepts for Anterior Cruciate Ligament Reconstruction: A Criterion-Based Rehabilitation Progression. J Orthop Sport Phys Ther 2012;42(7):601-614. doi:10.2519/jospt.2012.3871
57 Kaplan Y, Witvrouw E. When Is It Safe to Return to Sport After ACL Reconstruction? Reviewing the Criteria. Sports Health. 2019;11(4):301-305. doi:10.1177/1941738119846502
58. Kimball J. USSA Return to Snow Following Rehabilitation. Presented at: Medical Emergencies in Skiing and Snowboarding; 2022.
59. Davies WT, Myer GD, Read PJ. Is It Time We Better Understood the Tests We are Using for Return to Sport Decision Making Following ACL Reconstruction? A Critical Review of the Hop Tests. Sports Med 2020;50(3):485-495. doi:10.1007/ s40279-019-01221-7
60. Millikan N, Grooms DR, Hoffman B, Simon JE. The Development and Reliability of 4 Clinical Neurocognitive Single-Leg Hop Tests: Implications for Return to Activity Decision-Making. J Sport Rehabil 28(5). doi:10.1123/jsr.2018-0037
61. Rosen AB, Choi JY, Anderson K, Remski LE, Knarr BA. Development, validity, and test-retest reliability of a new neurocognitive functional performance test: The choice-reaction hop test. Phys Ther Sport 2023;59:80-84. doi:10.1016/j.ptsp.2022.12.003
62. Farraye BT, Simon JE, Chaput M, Kim H, Monfort SM, Grooms DR. Development and Reliability of a Visual-Cognitive Reactive Triple Hop Test. J Sport Rehabil. 2023;32(7):802-809. doi:10.1123/ jsr.2022-0398
63. Farraye BT, Chaput M, Simon JE, Kim H, Grooms DR, Monfort SM. Development and reliability of a visual-cognitive medial side hop for return to sport testing. Phys Ther Sport 2022;57:40-45. doi:10.1016/ j.ptsp.2022.07.004
64. Dingenen B, Truijen J, Bellemans J, Gokeler A. Test-retest reliability and discriminative ability of forward, medial and rotational single-leg hop tests. Knee 2019;26(5):978-987 doi:10.1016/ j.knee.2019.06.010
65. Zorko M, Nemec B, Babič J, Lešnik B, Supej M. The Waist Width of Skis Influences the Kinematics of the Knee Joint in Alpine Skiing. J Sports Sci Med 2015;14(3):606-619.
66. Niederer D, Giesche F, Janko M, et al. Unanticipated jump-landing quality in patients with anterior cruciate ligament reconstruction: How long after the surgery and return to sport does the reinjury risk factor persist? Clinical Biomechanics 2020;72:195-201. doi:10.1016/ j.clinbiomech.2019.12.021
67 Giesche F, Engeroff T, Wilke J, Niederer D, Vogt L, Banzer W Neurophysiological correlates of motor planning and movement initiation in ACLreconstructed individuals: a case–control study BMJ Open. 2018;8(9):e023048. doi:10.1136/ bmjopen-2018-023048
68. Chaaban CR, Turner JA, Padua DA. Think outside the box: Incorporating secondary cognitive tasks into return to sport testing after ACL reconstruction. Frontiers in Sports and Active Living 2023:4. doi:10.3389/fspor.2022.1089882
69. Draovitch P, Patel S, Marrone W, et al. The Return-to-Sport Clearance Continuum Is a Novel Approach Toward Return to Sport and Performance for the Professional Athlete. Arthrosc Sports Med Rehabil 2022;4(1):e93-e101. doi:10.1016/ j.asmr.2021.10.026
Robert C. Manske, PT, DPT, MEd, SCS, ATC, CSCS, FAPTA
Michael Voight, PT, DHSC, SCS, OCS, ATC, CSCS, FAPTA
Chris Wolfe, PT, DPT, OCS, Cert MDT
Phil Page, PT, PhD, ATC, CSCS, FACSM
Abstract
The rotator cuff, comprising the subscapularis, supraspinatus, infraspinatus, and teres minor muscles, plays a crucial role in stabilizing the glenohumeral joint by securing the head of the humerus within the glenoid cavity of the scapula. The tendinous insertions of these muscles generate tension within the capsule, enhancing joint stability during muscular activity. The rotator cuff is susceptible to damage from disease, injury, or trauma, which can result in tears or ruptures of one or more tendons. The evaluation of the infraspinatus muscle and tendon is vital for diagnosing and managing various shoulder pathologies. Accurate imaging to determine the specific muscle involvement and injury severity significantly impacts treatment decisions. Diagnostic musculoskeletal ultrasound (MSK-US) has emerged as a valuable tool for assessing the infraspinatus muscle and tendon, offering real-time, dynamic assessment capabilities essential for precise diagnosis and effective rehabilitation planning. This article reviews the utility and advantages of MSK-US in evaluating the infraspinatus muscle and tendon, emphasizing technique specifics, diagnostic accuracy, and comparative efficacy against other imaging modalities. It details a systematic approach to the ultrasound examination technique for the infraspinatus, including patient positioning and identification of common pathologies such as tears, tendinopathy, and calcifications. With recent advancements in transducer strength, image resolution, and operator training, ultrasound serves as an excellent alternative imaging modality for diagnosing rotator cuff tears. This article aims to equip rehabilitation professionals with a comprehensive understanding of MSK-US as a diagnostic tool for the infraspinatus, promoting more precise diagnosis, treatment planning and improved patient outcomes.
Introduction
Rotator cuff tears are a prevalent source of debilitating shoulder pain and reduced mobility, often leading to irreversible damage of the glenohumeral joint.1 They constitute a significant portion of shoulder-related disabilities in the United States, accounting for 30% to 70% of shoulder pain cases and representing a primary reason for over 4.5
million annual medical consultations.2-4 The progression of these tears typically initiates with partial-thickness damage and can advance to complete tendon rupture due to various pathological or traumatic factors. Symptoms commonly include nocturnal pain, shoulder weakness, and difficulty performing overhead activities.
The infraspinatus muscle, integral to the rotator cuff, frequently sustains injury in both athletic and general populations. The infraspinatus plays a primary role in facilitating external rotation motion. Equally important, the infraspinatus serves as a stabilizer of the glenohumeral joint. Together with other rotator cuff muscles, the infraspinatus reinforces the inherently fragile glenohumeral capsule, ensuring the humeral head remains securely positioned within the scapular glenoid cavity.
Disorders affecting the infraspinatus can significantly impair shoulder function and are commonly encountered in clinical practice. Accurate assessment of rotator cuff pathology is essential for effective management. Historically, contrast arthrography was pivotal in diagnosing full-thickness tears, yet its utility in assessing tear size and detecting partial-thickness tears is limited.5,6 Magnetic resonance imaging (MRI), though widely accepted, faces challenges in reliably distinguishing between tear types and cuff degeneration.6 Recently, MSK-US has gained prominence for its real-time imaging capabilities, safety profile, and cost-effectiveness in diagnosing infraspinatus tendon and muscle conditions.7 This article examines the role of MSK-US in the comprehensive evaluation of these structures, particularly relevant to rehabilitation professionals.
A comprehensive understanding of anatomy is essential for precise MSK-US evaluation, facilitating the identification of pathological alterations and the formulation of effective rehabilitation protocols. Situated beneath the scapular spine within the infraspinatus fossa, the infraspinatus muscle originates from this fossa and inserts into the greater tubercle of the humerus. Its fibers extend
towards the glenohumeral joint, attaching just inferior to the supraspinatus insertion. Primarily responsible for external rotation, horizontal abduction, and abduction of the humerus, the infraspinatus muscle collaborates with other rotator cuff muscles to stabilize the humeral head within the glenoid cavity. Innervated by the suprascapular nerve and predominantly vascularized by branches from the posterior humeral circumflex and suprascapular arteries, it exhibits comparatively robust vascular support, potentially reducing the incidence of pathology and degenerative changes. Patients experiencing an infraspinatus tear often report pain during resisted external rotation and active elevation movements.
Benefits of MSK-US
MSK-US offers several advantages over other imaging modalities in the assessment of the infraspinatus muscle and tendon. These include
•. Real-time Imaging: Allows for dynamic assessment during motion and muscle activation. This ability is crucial for identifying subtle dysfunctions that occur during movement, which are often missed by static imaging techniques.
•. Cost-Effectiveness: MSK-US is generally more affordable than MRI or CT scans, making it accessible for routine clinical use.
•. Non-Invasive: The procedure is non-invasive and welltolerated by patients, reducing the need for anesthesia or contrast agents.
•. High Resolution: Detailed visualization of soft tissue structures is possible at a higher resolution than MRI in some cases.
•. Immediate Results: MSK-US provides immediate feedback, allowing for quicker clinical decision-making. The immediate feedback and interactive nature of the ultrasound examination also facilitate enhanced patientclinician communication.
•. Accessibility and Safety: MSK-US is a patient-friendly option, avoiding the discomfort and contraindications associated with ionizing radiation and magnetic fields.
Limitations and Considerations
Despite its benefits, MSK-US also presents several limitations:
•. Operator Dependency: The accuracy of MSK-US is highly dependent on the skill and experience of the operator.
•. Limited Field of View: While excellent for soft tissues, MSK-US may not be as effective for evaluating bony structures.
•. Artifact Presence: Artifacts can sometimes obscure the view and complicate the interpretation of images.
MSK-US Technique for Evaluating the Infraspinatus
The examination of the infraspinatus muscle and tendon via MSK-US requires precise technique. Proper patient positioning and transducer placement are crucial to obtain accurate and reproducible images. The patient is typically positioned in a sitting posture with the back exposed and the arm in internal rotation to maximize infraspinatus visibility. The transducer is placed in a longitudinal and transverse orientation along the course of the muscle and tendon to assess for any structural abnormalities, such as tears, tendinopathy, or atrophy. Special attention is paid to the echogenicity and texture of the tendon, signs of inflammation, and the presence of bursal fluid.
•. Transducer: A high-frequency linear transducer (7–15 MHz) is typically used for evaluating the infraspinatus.
• Patient Positioning: The patient is usually seated position with the shoulder in slight abduction and internal rotation to optimize visualization of the infraspinatus.
•. Scanning Technique:
1. Transverse Scans: Begin with transverse scans to identify the spine of the scapula and follow the muscle belly towards its insertion on the humerus. The transducer is transversely placed inferiorly and slightly laterally from the scapular spine. Passive internal and external rotation of the patient’s arm may be helpful in visualization of the tendon. The infraspinatus tendon appears as a beak-shaped softtissue structure that progressively thins as it approaches its attachment to the posterior aspect of the greater tuberosity.6 Additional structures seen at this level are the posterior glenoid labrum, imaged as a hyperechoic triangular structure and the hypoechoic articular cartilage of the humerus.
2. Longitudinal Scans: Longitudinal scans help in assessing the tendon and its attachment to the greater tubercle of the humerus.
Key Findings
Ultrasound imaging of the infraspinatus must be carefully interpreted. Normal anatomy appears as a fibrillar pattern with uniform echotexture. Pathological findings may include
• Muscle Atrophy: Reduced muscle bulk can indicate chronic rotator cuff pathology.
•. Tendinopathy: Characterized by hypoechoic (darker) areas and tendon thickening which may signify tendinopathy or degeneration.
•. Tears: Full-thickness or partial-thickness tears are displayed as hypoechoic or anechoic (absence of echoes) defects within the tendon. Additionally, they may be identified by discontinuities in the tendon fibers.
•. Calcific tendinitis: Identified by hyperechoic (brighter) foci with or without acoustic shadowing.
Conclusion
Musculoskeletal ultrasound has emerged as a pivotal tool for assessing and managing disorders of the infraspinatus muscle and tendon. Its capability to deliver real-time, highresolution imaging renders it invaluable for dynamic evaluations and guiding rehabilitation protocols. Despite inherent limitations, the benefits of MSK-US position it as a cornerstone of contemporary clinical practice. To effectively integrate MSK-US into rehabilitation settings, practitioners must acquire specialized training encompassing both ultrasound techniques and detailed knowledge of infraspinatus musculoskeletal anatomy. By leveraging this technology in clinical practice, rehabilitation providers can elevate diagnostic accuracy, optimize therapeutic interventions, and ultimately enhance outcomes for patients grappling with shoulder pathologies.
References
1. Nathani A, Smith K, Wang T. Partial and full-thickness RCT: modern repair techniques. Curr Rev Musculoskelet Med. 2018;11(1):113-121.
2. Mitchell C, Adebajo A, Hay E, et al. Shoulder pain: diagnosis and management in primary care. BMJ. 2005;331(7525):1124.
3. Rees JL. The pathogenesis and surgical treatment of tears of the rotator cuff. J Bone Joint Surg Br. 2008;90(7):827-832.
4. Holsbeeck MT, Mack LA, Matsen FA, et al. The rotator cuff. In: Rumack CM, Wilson SR, Charboneau JW, eds. Diagnostic Ultrasound. Vol 1. 2nd ed. Mosby-Yearbook, St. Louis MO; 1998:843–861.
5. Holling A. Sonography of the rotator cuff: an overview. J Diagn Med Sonog. 2001;17:144-150.
6. Mack LA, Matsen. The rotator cuff. In: Fornage BD, ed. Musculoskeletal Ultrasound. Churchill Livingston, New York; 1995:113–133.
7. Jacobson JA. Musculoskeletal sonography and MR imaging: a role for both imaging methods. Radiol Clin North Am. 1999;37:713–735.

Figure 1A: Patient Position
The patient is seated with the ipsilateral hand resting on the contralateral shoulder. This places the arm in a slight internal rotation and adduction pulling the infraspinatus in an anterior-lateral tensioned position. This provides the ability to see abnormalities in the tendon.
Figure 1B: Short Axis Transducer Placement
Short Axis (SAX) view places the transducer perpendicular to the floor, parallel to the axial spine and is perpendicular to the tendon fibers as they insert onto the humeral head. This view provides the ability to image the tendon footprint at its distal insertion.
Figure 1C: Long Axis Transducer Placement
Long Axis (LAX) view places the transducer parallel to the floor and perpendicular to the axial spine. This view places the transducer parallel with the fibers of the infraspinatus tendon. Start with the transducer placed in the middle of the deltoid and capturing the edge of the acromion. The acromion will be a bony landmark for orientation along with the humeral head.
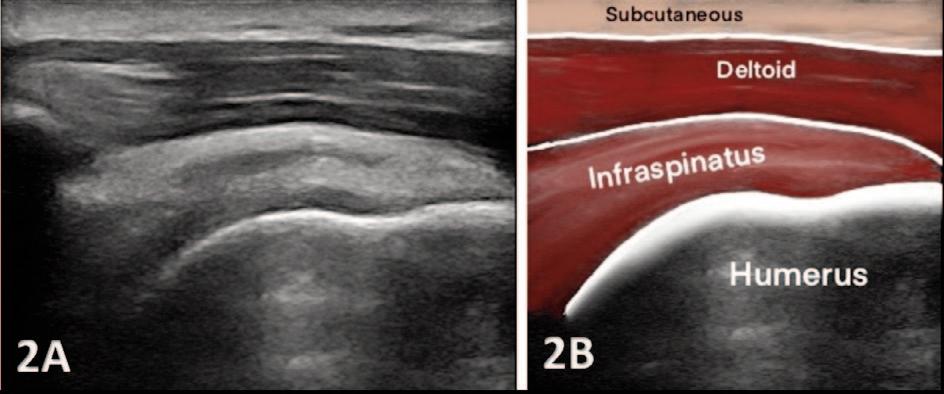
Figures 2A and 2B Short Axis View: Look for the bony cortex of the humeral head initially and the parallel fibers of the infraspinatus tendon as it inserts on the lateral facet of the greater tuberosity. The mild indention noted in the middle of the bony cortex represents the anatomical neck. The infraspinatus tendon lies on the humeral head and maintains a uniform appearance. Note that the tendon will exhibit a hyperechoic brightness.Identify the humeral head, the hyaline cartilage (seen as an anechoic interface above the cortex), the hyperechoic infraspinatus tendon, and its anechoic insertion onto the lateral facet of the greater tuberosity. This insertion typically has a larger anechoic footprint at its distal insertion, which should not be mistaken for pathology. Superior to the tendon is the anechoic space for the bursa, typically visible in nonpathological infraspinatus tendons. Above the tendon and bursa, you will see the deltoid muscle and the subcutaneous fat tissue at the top of the image.

Figures 3a and 3b Long Axis View. The Long Axis View technique will be parallel to the spine of the scapula placing the transducer parallel with the tendon fibers. This position should capture the edge of the acromion as shown in image 2A above. The acromion is the anechoic structure on the left and will be a bony landmark for orientation. Superior to the tendon would be the bursa and deltoid muscle followed by the subcutaneous fat tissue. The infraspinatus tendon should have a consistent hyperechoic nature but slightly less hyperechoic compared to the supraspinatus tendon..
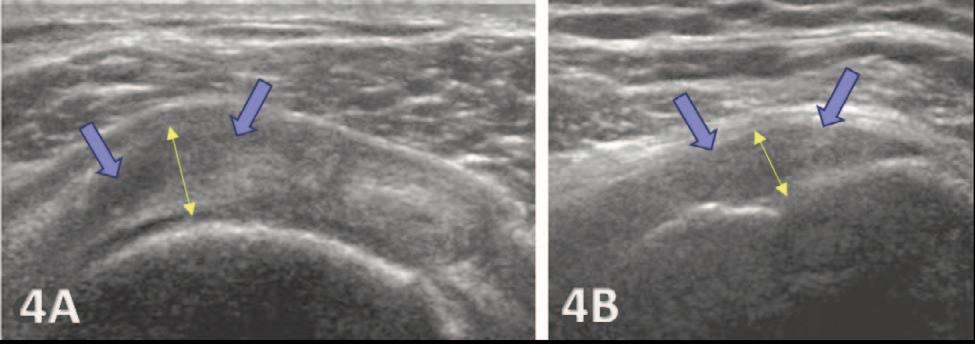
Figures 4a and 4b: Infraspinatus tendon in Short Axis view and Long Axis view shows increased thickness. This thickness is greater than 4.2 mm (outlined in yellow) and is known to indicate increased cellularity and is one of the criteria that is used to diagnosis tendinosis. Normal tendon thickness can vary slightly with age and with dominant verses non-dominant shoulders. For an individual 30-39 years old, normal thickness is 4.2 + 0.7 mm. Both images show abnormal hypoechogenicity (blue arrows) along with increased tendon thickness (yellow arrows).
TENDINOUS FAILURE IN SHORT AXIS (SAX) VIEW
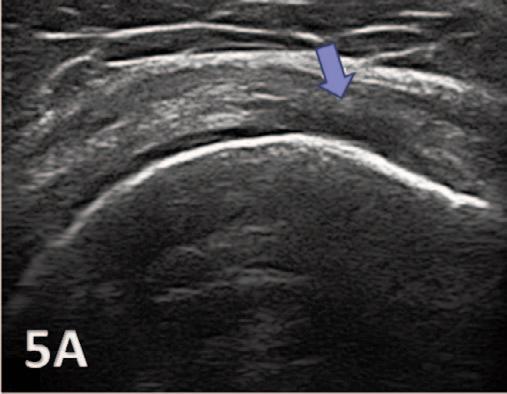
Figure 5a: Disruption of the infraspinatus tendon which is shown above. Blue Arrow pointing to the hypoechoic changes within the tendon.
TENDON CALCIFICATION IN SHORT AXIS (SAX)

Figure 6a: Tendon Calcification and degenerative tendon. Ultrasound image shows a well-defined linear calcific deposit (blue arrows) along the infraspinatus tendon fibers with partial shadowing (yellow arrow).