DRUG DELIVERY
DEVELOPING A SMART MEDICAL DEVICE IS A TASK THAT LIES SOMEWHERE BETWEEN COMPLICATED AND COMPLEX. STRICT REGULATIONS AND MULTILAYERED REQUIREMENTS NECESSITATE AN ARRAY OF SKILLS FROM PRODUCT DESIGN TO MANUFACTURING AND PROCESSING TO RISK MANAGEMENT. PEIK-CHRISTIAN WITTE, HEAD OF ENGINEERING & INNOVATION, SANNER GMBH, EXPLAINS HOW TO DEVELOP INTELLIGENT PRODUCTS WITH DESIGN FOR EXCELLENCE.
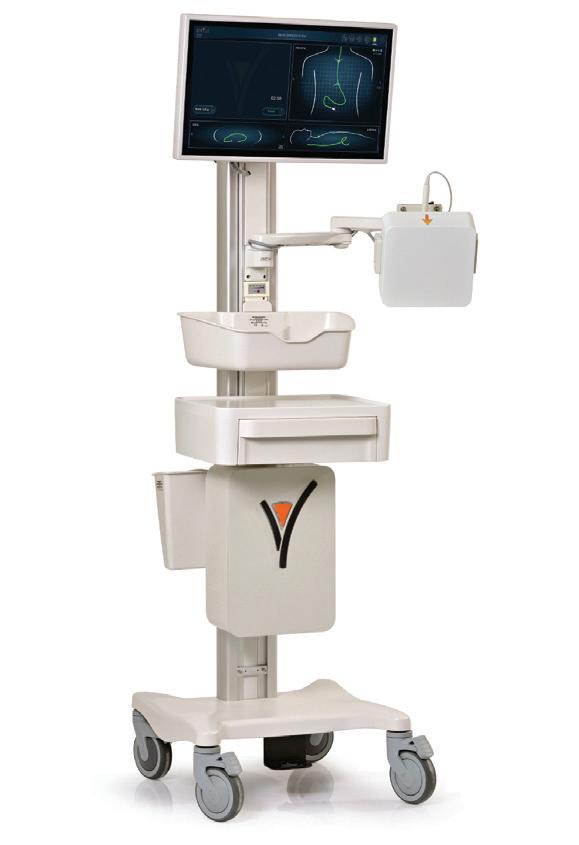
THE X FACTOR mart medical devices with certain functions such as reminders or dosage can increase therapy adherence. Products range from intelligent tablet dispensers to retrofittable add-ons for inhalers or injection systems.
S
DESIGN FOR PRODUCT Requirements management already plays an important role in the initial development phase. Based on numerous customer requirements, product designers should develop concepts that incorporate functionality, manufacturability and costs — also for subsequent series production — and physically convert the results in CAD (computer-aided design) models.
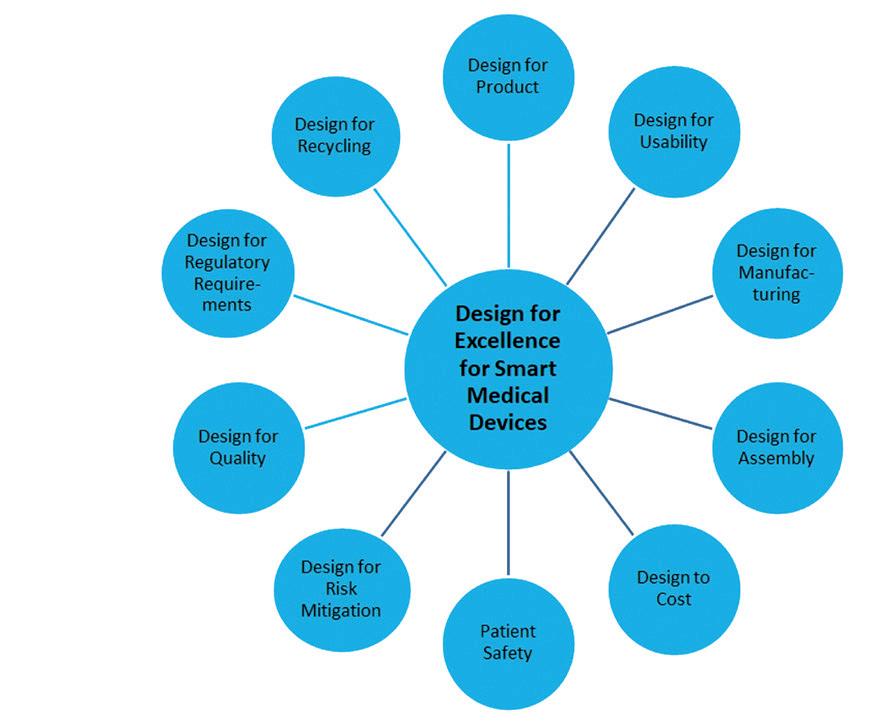
Numerous factors feed into the development and subsequent production of such products. Each factor has a different emphasis depending on the complexity and design of the device. In Design for Excellence (DfX), the X stands for a variable that makes a development successful only in optimal interaction with the others (Figure 1).
DESIGN FOR USABILITY User-friendliness and ergonomics make decisive contributions to the correct use and acceptance of the smart medical device, which ultimately improves the therapeutic success. Particular attention must therefore be paid to usability with the aim of minimizing risks from application errors, ensuring patient safety, and creating the framework conditions for the highest possible level of adherence. DESIGN FOR MANUFACTURING The product must be optimally designed at an early stage regarding the planned manufacturing and assembly conditions, quality, and costs. Particularly in the case of plastic components, product and process development must go hand in hand, hence material selection, tooling and injection molding technology, the lowest possible number of components and functional integration are considered. DESIGN FOR ASSEMBLY The product design, including product structure, is optimized with regard to assembly. The assembly concept (that is, a partially versus a fully automated process) depends on the number of parts to be assembled. DESIGN TO COST An optimum cost–benefit ratio and low follow-up and modification costs are also relevant. Considerations include the optimum use of materials, the lowest possible number of components, and optimizing manufacturing process cycle times. As electronic components for smart medical devices can also boost manufacturing costs, these too warrant detailed analysis.
Figure 1: According to the Design for Excellence (DfX) strategy, only the optimal interaction with numerous other variables will lead to success.
8
DESIGN FOR PATIENT SAFETY Patient protection, patient well-being and patient benefits are important issues in the development, production and application of smart medical WWW.MEDICALPLASTICSNEWS.COM