15 minute read
Department of Bioengineering, McGowan Institute for Regenerative Medicine, Renerva, LLC
Assessment of luminal fillers in nerve guidance conduits for promoting regeneration following peripheral nerve injury
Carol Vinskia, Tyler Medera,b, Bryan Brown, Ph.D.a,b,c
aDepartment of Bioengineering, bMcGowan Institute for Regenerative Medicine, c Renerva, LLC
Carol Vinski Carol Vinski is a native Pittsburgher. Her passion for the improvement of medicine and healthcare motivates her to become a biomechanical engineer in the field of prosthetics.
Dr. Bryan Brown is an Associate Professor in the Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute at the University of Pittsburgh. He is also a Bryan Brown, Ph.D. core faculty member of the McGowan Institute for Regenerative Medicine where he serves as the Director of Educational Outreach. Dr. Brown is also an Adjunct Assistant Professor of Clinical Sciences at the Cornell University College of Veterinary Medicine and Chief Technology Officer of Renerva, LLC, a Pittsburgh-based start-up company.
Significance Statement
Autologous grafting, the current clinical standard to treat peripheral nerve injury (PNI), often results in unsatisfactory nerve healing and always causes a loss of feeling at the donor site. This study analyzes the most effective laboratory nerve guidance conduits and luminal fillers to act as an alternative solution to autografting.
Category Review Paper
Keywords: luminal fillers, nerve guidance conduits,
nerve repair, biomechanics of nerves
Abstract
Peripheral nerve injury (PNI) is a growing field of scientific research, and transected PNIs are difficult to repair due to the quick buildup of scar tissue blocking axon regeneration. Autologous grafting, the clinical standard of care, can cause loss of feeling at the donor site, and patients may not have enough viable donor tissue to complete this procedure depending on injury severity. To offer an effective alternative to autografting without loss of sensation, nerve guidance conduits (NGCs) are being explored but are not as successful as the clinical standard. Additives to NGC lumen (luminal fillers) are being researched to obtain results at least as successful as the autograft without its limitations. As of yet, no luminal fillers have entered into clinical use. The Brown Lab has previously developed a luminal filler derived from Porcine sciatic Nerve extracellular Matrix (PNM). To better understand how PNM performs in the context of NGC additives as a whole, the present study primarily examines reports of various luminal fillers to determine what characteristics predict success as a result of their application. Secondly, reports analyzing Schwann cell (SC) activity under variable biomechanical stimuli were considered as luminal fillers to provide a mechanical substrate which interacts with SCs. Overall, successful luminal fillers most often contained highly porous and permeable substrates with steep mechanical gradients for maximum SC activity. By utilizing this knowledge, successful luminal fillers and their respective conduits can be used to efficiently heal PNIs to maximize functional returns in patients.
1. Introduction
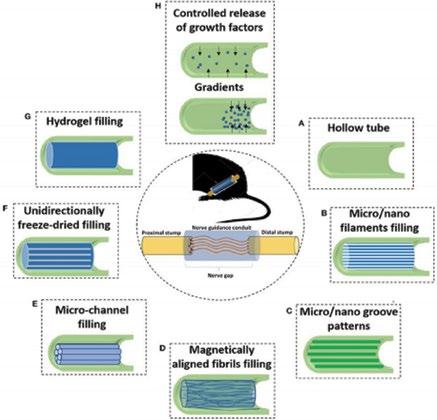
Occurring in 3% of traumas, peripheral nerve injury (PNI) often requires intervention. Since only 50% of surgical repairs demonstrate a satisfactory recovery rate [1,2], this field of scientific research is growing quickly. Upon injury, development of scar tissue can rapidly outpace axon regrowth within the nerve, preventing functional returns and resulting in unsatisfactory PNI healing [3]. The clinical standard for repairing PNIs is using an autologous nerve graft from the patient’s own body to bridge the severed endings of the damaged nerve, but this process causes a loss of sensation in the donor site of the body and is not always an option for patients [4]. To offer an effective alternative to autografting, nerve guidance conduits (NGCs) have been developed; however, they are not as successful as autografts and thus have a reduced capacity to heal PNIs [5]. To improve their efficacy, NGCs are being explored in combination with additives within the lumen (luminal fillers) to provide a therapy of equal or greater value than autografting; examples of these are shown in Fig. 1.
Figure 1. NGCs and luminal fillers. In the center of the image, the nerve guidance conduit can be seen between the proximal and distal ends of the PNI. The hollow tube is the NGC (A), and it is filled with luminal fillers of aligned fibers (D, F), micro fillings (B, E), or hydrogel (G). The NGC is given grooves (C) or allows controlled release of NGFs via pore size or gradients (H).
However, no luminal fillers have entered into clinical use yet. The Brown Lab has previously developed a luminal filler derived from Porcine sciatic Nerve extracellular Matrix (PNM) [6]. To better understand how PNM performs in the context of NGC additives as a whole, the present study examines reports of various luminal fillers to determine which characteristics predict success as a result of their application. Important factors to consider while creating an NGC are the mechanics of the resting (quiescent) and activated nerve and Schwann cells (SCs), both of which contribute to peripheral nerve regrowth. As shown in Fig. 2, SCs are imperative to the regrowth of peripheral nerves.

Figure 2. Peripheral nerve regrowth after PNI. At the injury site, the distal end of the neuron breaks down all the way as the proximal end of the neuron breaks down a little bit. Next, SCs help the neuron vasculature to replenish, creating the Bands of Bungner. Finally, the axon regenerates along these mature vascular bridges.
This process of transitioning between the rest and active states is due to transcriptional regulators, such as c-Jun, an activator of “a repair program to support [nerve] regeneration” [7]. SCs are regulated by MAPK and other signaling systems as well, but substrate stiffness also plays an important role in SC transition between quiescence and acquiescence [7]. The purpose of this review article is to summarize research studies that focus on mechanical properties of the materials, to develop better understanding of the environment in which peripheral nerves live, and to promote manipulation of substrates in healing PNIs via NGCs and luminal fillers.
2. Methods
Papers were found in the Google Scholar database, using keywords and phrases such as “luminal fillers,” “peripheral nerve injury conduits,” “peripheral nerve regrowth,” and “Schwann cell growth and biomechanics.”
2.1 Luminal Fillers Papers
Studies applying luminal fillers in animal models were selected to compare their contributions towards nerve healing. Key properties, such as material choice and filler porosity or addition of SCs and/or neuronal growth factors (NGFs), were compared from these luminal fillers. All papers used can be found in Table 1.
Studies NGC and Filler Properties General Success
[2], [4], [6], [25], [30] aligned fiber orientation in conduit or luminal filler successful for travel of nutrients/waste
[12], [32], [33], [34] NGFs in luminal filler successful for improving PNI healing
[30] unaligned fiber orientation in luminal filler unsuccessful, made it harder to transport nutrients/waste and for SCs to travel on
[2], [7], [13], [20], [23], [25], [29], [31], [33]
[1], [23], [25], [27], [33], [34] [17], [11], [26]
[4], [6], [5], [9], [10], [12], [14], [17], [18], [23], [29] [1], [4], [3], [7], [9], [11], [12], [13], [14], [15], [21], [22], [25], [30] [3], [6], [8], [11], [13], [14], [17], [19], [22], [24], [26], [29], [30], [34] conduit porosity
more successful with pore sizes between 10 and 40 µm luminal filler porosity generally more successful with pores than without
PNM in conduit or luminal filler more successful when PNM is used as the sole luminal filler
SCs added to conduit or luminal filler
collagen in conduit or luminal filler
type of gel or gellan gum within conduit or luminal filler more successful when aligned more successful when collagen is used as a supportive structure more successful when gel is broken down to allow for controlled release of SCs/NGFs/etc.
[26], [28], [29] PCL in conduit and/or more successful when luminal filler paired with factors that contribute to SC proliferation or PNI healing [1] Aigner, T. B. et al (2020). doi:10.1016/j.mtbio.2020.100042 [2] Cai, J. et al (2005). doi:10.1002/jbm.a.30432 [3] Carriel, V. et al (2013). doi:10.1088/1741-2560/10/2/026022 [4] Ceballos, D. et al (1999). doi:10.1006/exnr.1999.7111 [5] Dietzmeyer, N. et al (2020). doi:10.1177/0963689720910095 [6] Du, J. et al (2017). doi:10.1016/j.actbio.2017.04.010 [7] Ezra, M. et al (2016). doi:10.1089/ten.tea.2015.0354 [8] Gnavi, S. et al (2014). doi:10.1002/term.1936 [9] Gu, Y. et al (2016). doi:10.1002/term.2123 [10] Kalbermatten, D. F. et al (2008). doi:10.1016/j.bjps.2007.12.015 [11] Kohn-Polster, C. et al (2017). doi:10.3390/ijms18051104 [12] Lackington, W. A. et al (2018). doi:10.1016/j.actbio.2018.06.014 [13] Lee, Y. S. et al (2016). doi:10.1002/jbm.a.35630 [14] Lohmeyer, J. A. et al (2007). doi:10.1177/039139880703000109 [15] Matsumoto, K. et al (2000). doi:10.1016/s0006-8993(00)02207-1 [16] Matsuura, S. et al (2006). doi:10.1016/j.urology.2006.09.051 [17] Meder, Tyler et al (2019). doi: 10.1002/jbm.a.36235 [18] Meyer, C. et al (2016). doi:10.3727/096368915x688010 [19] Nakayama, K. et al (2007). doi:10.1111/j.1525-1594.2007.00418.x [20] Nectow, A. R. et al (2012). doi:10.1089/ten.teb.2011.0240 [21] Newman, K. D. et al (2006). doi:10.1177/039139880602901109 [22] Pace, L. A. et al (2013). doi:10.1089/ten.tea.2013.0084 [23] Rao, J. et al (2017). doi:10.1016/j.msec.2016.12.085 [24] Roldán-Carmona, Cristina, et al (2014). doi: 10.1039/x0xx00000x [25] Ryan, A. J. et al (2017). doi:10.1002/adhm.201700954 [26] Sun, A. X. et al (2019). doi:10.1016/j.biomaterials.2019.01.038 [27] Sun, B. et al (2017). doi:10.1021/acsami.7b06707 [28] Tonda-Turo, C. et al (2011). doi:10.1002/adem.201080099 [29] Tonda-Turo, C. et al (2014). doi:10.1002/term.1902 [30] Verdú, E. et al (2002). PMID:12515893 [31] Wang, X. et al (2005). doi:10.1093/brain/awh517 [32] Wood, M. D. et al (2009). doi:10.1016/j.actbio.2008.11.008 [33] Wu, H. et al (2018). doi:10.1016/j.jmbbm.2017.10.031 [34] Zhang, L. et al (2016). doi:10.1007/s11595-016-1545-y Table 1: All References for Luminal Fillers 2.2 C Biomechanics Papers
Studies examining SCs in response to mechanical cues were selected to find the ideal range of luminal filler mechanical properties that were conducive to PNI healing. Studies concerning SC mechanics and how SCs react to the substrate surrounding them were selected from in vitro studies with comparisons made between Young’s moduli, substrate stiffness/compliance, and mechanical gradients. All papers used can be found in Table 2.
Studies Discussion
[3], [8], [12] [5], [6], [7], [10], [13] impact of altering Young's modulus mechanical properties of the microenvironment of healthy and injured peripheral nerves
[1], [2], [4], [11], [14] Schwann cell growth and quiescence patterns [1] Boerboom, A. et al (2017). doi:10.3389/fnmol.2017.00038 [2] Evans, E. et al (2018). doi:10.1186/s40824-018-0124-z [3] Gittes, F. (1993). doi:10.1083/jcb.120.4.923 [4] Gu, Y. et al (2012). doi:10.1016/j.biomaterials.2012.06.006 [5] Hunt, G. C. (2002). doi:10.1179/108331902125001888 [6] Janmey, P. A. et al (2019). doi:10.1152/physrev.00013.2019 [7] Ju, M. S. et al (2017). doi:10.1299/jbse.16-00678 [8] Manssor, N. A. S. et al (2016). doi:10.1159/000431328 [9] Meder, Tyler et al (2019). doi: 10.1002/jbm.a.36235 [10] Pfister, B. J. et al (2020). doi:10.1016/j.cobme.2020.05.009 [11] Rosso, G. et al (2017). doi:10.3389/fnmol.2017.00345 [12] Rosso, G. et al (2019). doi:10.1063/1.5108867 [13] Sundararaghavan, H. G. et al (2009).doi:10.1002/bit.22074 [14] Xu, Z. et al (2019). doi:10.1002/term.2987 Table 2: All References for Schwann Cell Biomechanics
3. Results
3.1 Luminal Filler
Using the selection criteria in 2.1, 33 studies were examined for luminal filler efficacy. Overall, luminal fillers were found to be effective at promoting nerve healing through increased SC migration or neurite extension when containing aligned fiber orientation or added NGFs. Since SCs travel along straight fibers during nerve healing, as seen in Fig. 2, aligned fibers must produce more successfully healed PNIs than luminal fillers containing densely packed or unaligned substrates, which had negative effects on PNI healing [8]. Similarly, NGFs are neuronal growth factors and thus improve nerve regrowth, so adding more NGFs to a luminal filler or NGC can only increase PNI healing. It was observed that key predictors between successful and unsuccessful luminal filler were fiber alignment and substrate porosity [9,10]. Pores in the luminal fillers allow nutrients and NGFs in and allow waste to flow out. If the pore size is too large, scar tissue infiltrates into the space where the nerve is trying to regrow and thus keeps the nerve from reconnecting and giving functional returns. If pores are too small, waste cannot leave and nutrients and NGFs cannot enter the area to allow PNI healing [11]. Thus, NGC porosity over long gap defects is a key determining factor in the success of tissue within the lumen; for these PNIs, ideal pore size ranged from 10 to 40 μm [12]. Some gap sizes, however, are small enough that they do not require pores for removal of waste and diffusion of nutrients and NGFs because such PNIs heal much faster
than larger gap sizes. Thus, small gap sizes do not require pore size to be a significant contributing factor in NGC and luminal filler success.
3.2 SC Biomechanics
From the 11 studies examining SCs with mechanical cues, SC function was found to be greatly affected by the Young’s moduli in the substrate surrounding them. In Gu’s study, the 1-3 day old rat sciatic nerves grew most successfully on a Young’s modulus of 7.45 kPa as opposed to 9.10 kPa or 12.04 kPa [13], and another study found that 8.67 kPa worked the best out of the other stiffnesses used [14]. Thus, an elastic modulus too high or low will result in less viable SCs, suggesting that there is a theoretical exact Young’s modulus value that will promote nerve healing better than any other number. In addition to specific Young’s moduli, SCs preferred travelling down steep gradients when they transitioned to their activated state as opposed to staying on a flat surface. The presence of the gradient pushed the SCs to their acquiescent state for PNI healing through increased spread, elongated nuclei, and increased speed and direction [15]. However, substrates continuously promoting SC activation revealed difficulty in termination of the activation response, making it difficult for the nerve to return to its quiescent state.
4. Discussion
4.1 Luminal Fillers
From 3.1, it can be inferred that the key materials for successful luminal fillers are aligned fibers over all gap sizes and specific porosity over large gap sizes. Overall, aligned fibers within NGCs and luminal fillers, as in Fig. 2.D,F, greatly improved the success of PNI healing. In addition, pore sizes large enough for nutrients to enter but small enough that scar tissue cannot invaginate into the NGCs and luminal fillers was imperative for success over large gaps. If the gap is small enough, porosity is not an issue due to the short amount of time it will take for the PNI to heal compared to the amount of time it takes for scar tissue to infiltrate the regenerating nerve.
4.2 SC Biomechanics
From 3.2, it can be inferred that it is theoretically best to use a material within the luminal filler that begins with a stiffness gradient to promote PNI healing but is remodeled over time to mechanically match native nerves and promote SC quiescence. This would best utilize SCs to help improve nerve regrowth, as seen in Fig. 2, while preventing SCs from having an elongated activation response.
5. Conclusions
The luminal filler should be used in combination with NGFs with pores of appropriate size and that allow exchange of waste, nutrients, and NGFs. When these criteria are not met, it is possible for a luminal filler to promote invagination of scar tissue or inhibit axon penetration, decreasing healing as a result. Successful luminal fillers most often contained highly porous and permeable substrates with either aligned fibers or added NGFs. Increased success could be observed with a steep initial gradient to promote SC activation and migration. As the luminal filler gradient degrades, natural tissue will deposit, leaving a suitable environment for SCs to return to their quiescent state. This process allows for increased functional returns. The conclusions made in this study could be pursued to improve patient healing of PNIs.
6. Acknowledgements
Funding was provided by the Swanson School of Engineering, the Department of Bioengineering, and members of the Brown Lab including PI, Dr. Bryan Brown, and my student mentor, Tyler Meder. Funding for the nerve project was provided by the Wallace Coulter Foundation and the PInCH program through the University of Pittsburgh.
7. References
[1] C. A. Taylor, D. Braza, J. B. Rice, T. Dillingham. The incidence of peripheral nerve injury in extremity trauma, Am J Phys Med Rehabil. 87(5) 381-5, 2008. [2] A Portincasa, G Gozzo, D Parisi, L Annacontini, A Campanale, G Basso, A Maiorella, Microsurgical treatment of injury to peripheral nerves in upper and lower limbs: a critical review of the last 8 years Microsurgery. 27(5) 455–462, 2007. [3] Ngeow et al, Scar less: a review of methods of scar reduction at sites of peripheral nerve repair, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 109(3) 357–366, 2010 [4] D. Grinsell, C. P. Keating, Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies, Biomed Res Int 2014. [5] Brattain. Magellan Medical Technology Consultants, Inc. p. 1-11, 2013. [6] T. A. Prest, E. Yeager, S. T. LoPresti, Nerve-Specific, Xenogeneic Extracellular Matrix Hydrogel Promotes Recovery Following Peripheral Nerve Injury, J Biomed Mater Res A. 106(2) 450-459, 2018.
[7] A. Boerboom,V. Dion, A. Chariot, R. Franzen, Molecular Mechanisms Involved in Schwann Cell Plasticity, Frontiers in Molecular Neuroscience, 2017. [8] Verdú E, Labrador RO, Rodríguez FJ, Ceballos D, Forés J, Navarro X, Alignment of collagen and laminin-containing gels improve nerve regeneration within silicone tubes, Restor Neurol Neurosci., 20(5):169-179, 2002. [9] Du, J., Liu, J., Yao, S., Mao, H., Peng, J., Sun, X., … Wang, X, Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel, Acta Biomaterialia, 55, 296–309, 2017. [10] Cai, J., Peng, X., Nelson, K. D., Eberhart, R., & Smith, G. M., Permeable guidance channels containing microfilament scaffolds enhance axon growth and maturation, Journal of Biomedical Materials Research Part A, 75A(2), 374–386, 2005. [11] Rao, J., Cheng, Y., Liu, Y., Ye, Z., Zhan, B., Quan, D., & Xu, Y., Materials Science and Engineering: C, 73, 319–332, 2017. [12] A. R. Nectow, K. G. Marra, D. L. Kaplan, Biomaterials for the Development of Peripheral Nerve Guidance Conduits, Tissue Engineering Part B: Reviews, 18(1), 40–50. [13] Gu, Y., Ji, Y., Zhao, Y., Liu, Y., Ding, F., Gu, X., Yang, Y, The influence of substrate stiffness on the behavior and functions of Schwann cells in culture, Biomaterials, 33(28), 6672–6681, 2012. [14] Xu, Z., Orkwis, J. A., DeVine, B. M., & Harris, G. M., Journal of Tissue Engineering and Regenerative Medicine, 2019. [15] Evans, E., Brady, S., Tripathi, A., Schwann cell durotaxis can be guided by physiologically relevant stiffness gradients, Biomater Res 22, 14 (2018).










