
16 minute read
Altered Feed Forward Inhibition of Striosomes is Linked to Aberrant Value Based Decision Making in Chronically Stressed Mice
Altered Feed-Forward Inhibition of Striosomes is Linked to Aberrant Value-Based Decision Making in Chronically
Jessica Jenkins
Advertisement
Individuals suffering from disorders such as anxiety and depression often exhibit difficulty with rational decision making. Since these disorders are frequently a consequence of chronic stressors, Friedman et al. (2017) hypothesized that stress may also be responsible for maladaptive changes in the neural circuitry involved in evaluative thinking and decision making. Specifically, their region of interest was the prefrontal corticostriatal circuit since previous research identified this area to be important for evaluation. A T-maze task was used to identify differences in value-based decision-making behavior (decisions based on rewards and costs) between chronically stressed rats and control. Compared to control, stressed rats demonstrated flawed cost-benefit evaluation, since they chose absolute highrisk high-reward options more frequently than alternatives with a maximum benefit-cost difference (maximum value). This discrepancy in behavior correlated with a decrease in the spike activity in the prefrontal-prelimbic cortex neurons (PFC-PL), which in return caused a decrease in the firing of synapsing fast-spiking interneurons (FSI) and an increase in the activity of projecting neurons in the striosome (SPN). Optogenetic excitation of the PFC-PL neurons during the T-maze task successfully rescued decision making in chronically stressed mice, increasing confidence that the regulation of striosome activity plays an important role in healthy executive function. Freidman and colleagues (2017) referred to previous literature that identified striosomal projections that synapse directly with subsets of dopaminergic neurons in the substantia nigra. These neurons respond to rewarding and aversive stimuli, therefore it is possible that stress-induced overactivity in the striosome may be causing the erratic responses to costs and rewards in the T-maze task. Future studies are encouraged to implement some changes in the design of the T-maze task to overcome confounding effect, and to further explore the effect of chronic stress on the connection between the striosome and its downstream projections to the substantia nigra.
Key words: chronic stress, cost-benefit evaluation, striosome, value-based decision making, prefrontalprelimbic cortex, striosomal projecting neurons, fast spiking interneurons, T-maze task, prefrontal corticostriatal circuit, feed-forward inhibition, foot shock, optogenetics
Introduction Chronic stress is one of the leading factors that cause anxiety and depressive disorders (Khan & Khan, 2017). According to the American Psychological Association, reported levels of stress are on the incline, with an average 6% annual increase in stress levels between 2012 and 2017 in the United States (Bethune & Lewan, 2017). Furthermore, a 2017 report by the APA put forth the alarming statistic that at a third of individuals experience symptoms of nervousness, headaches and feelings of depression due to the stressors in their lives (Bethune & Lewan, 2017).
Impairment in cognitive functions such as attention, memory and evaluative thinking are common symptoms of both anxiety and depression according to the Diagnostic and Statistical Manual of Mental Disorders, 5 th Edition (2013). Experimental evidence for this was provided through a case-control study, whereby individuals with Major Depressive Disorder performed significantly worse in value-based decision tasks that involve punishment and reward learning. (Mukherjee et al., 2020). Previous repeated trans-magnetic stimulation studies on humans and lesion studies with macaque monkeys have collected evidence for the importance in the connectivity within the prefrontal, orbitofrontal and prelimbic systems during reward-guided learning and decision making (Amemori & Graybiel, 2012). In 2015, Friedman and colleagues’ discovery of prefrontal corticostriatal circuit lead to their suggestion that this circuitry likely contributes to the executive control of value-based decision making, since it facilitates the recruitment of the reward system during evaluation (Friedman et al., 2015). Given the compilation of the research listed, it is possible that the stress-inflicted impairment of value-based decision making is brought by changes in the connectivity of the prefrontal corticostriatal circuit. Nonetheless, until Friedman and colleagues’ 2017 study, there was no concrete evidence to suggest that these changes could be brought by stress, and nor was there a clear understanding of how these changes bring upon maladaptive decision-making. To address these questions, Friedman et al. (2017) chose three components of interest within prefrontal corticostriatal circuit, to carry out electrophysiological measurements during a T-maze cost-benefit conflict decision task. The prefrontal corticostriatal circuit was implicated by previous research to be important for cost, effort and reward evaluation (Amemori & Graybiel, 2012). This was done to compare neuronal activity levels in chronically stressed rats versus control during value-based decisionmaking. In doing so, it was confirmed that there were differences in brain activity between the two groups of rats, and these differences correlated with stress-induced impairment of cost-benefit evaluation in stressed rats. The reduction in the feed-forward inhibition of striosome projecting neurons was of particular interest to the authors, since these projections synapse with neurons in the substantia nigra that regulate motivated behaviors guided by reward and aversion (Sousa & Almeida, 2012). Confidence in these results was increased when optogenetic intervention successfully rescued stress-induced impairments in decision making.
MAJOR RESULTS Experimental rats underwent 14 days of foot shock as chronic stress, and then were tested in a costbenefit T-maze task. The cost-benefit T-maze task is an assay commonly used in psychology and neuroscience research to study reward-based decision making (Cousins et al., 1996; Schweimer & Hauber, 2006). Friedman et al. (2017) used fluorescent light as an aversive stimulus and chocolate milk as a reward. Different combinations of light intensity and chocolate milk concentration were presented to the rat at the left and right arm of the T-maze, to vary the size of the costs and rewards at each arm across trials. This was done to prompt the rats to evaluate which option was worth choosing, given the size of the combined reward and cost at each arm. The experiment was repeated after the rats were fitted with electrodes to measure spike activity at the PFCPL, FSI and SPN. After stress-induced spike activity changes were identified, optogenetic manipulation was introduced at the PFC-PL of stressed rats to counteract the effect of chronic stress on behavior. 14 days of chronic stress causes changes in responsiveness to costs and rewards in a T-Maze cost-benefit conflict task.
HIGH
1B
LOW

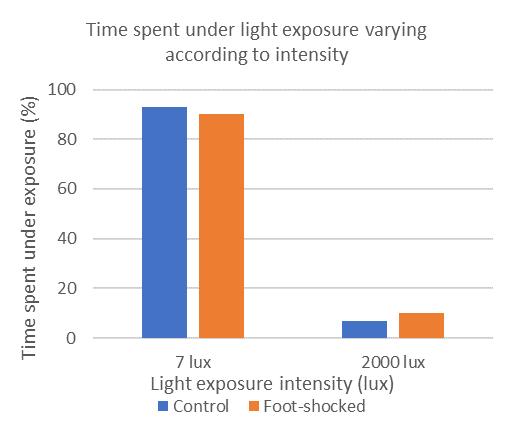
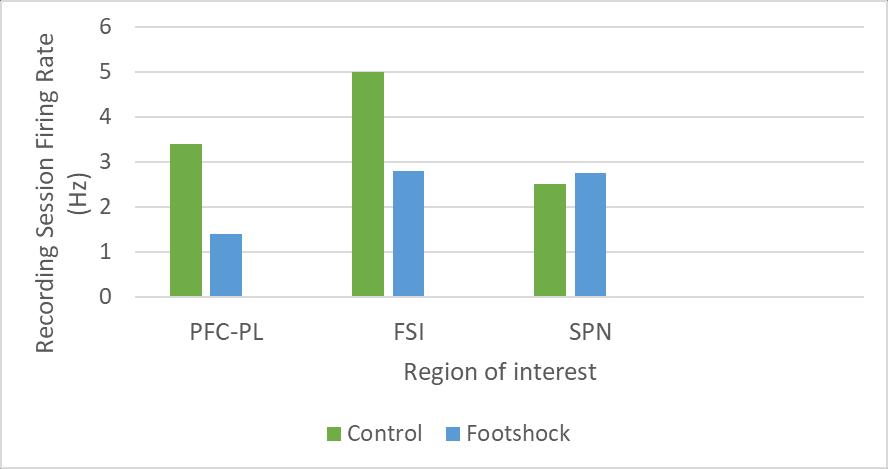
Figure 2: Changes in the prefrontal corticostriatal circuit activity following 14 days of chronic stress: spike activity in the PFCPL, FSI and SNP.
Spike activity recorded during the T-maze test revealed that chronically stressed rats had lower PFC-PL and FSI neural activity measures compared to control, p < 0.01 (KolmogorovSmirnov test and t test). In contrast, neural activity in the SPN was greater in chronically stressed mice, compared to control p < 0.01 (Kolmogorov-Smirnov test and t test). These differences were speculated to cause the stress-induced behavioral changes in value-based decision-making. Optogenetic rescue of value-based decision-making behavior in chronically stressed rats.
1C
3A 3B
Figure1: T-maze experiment investigates cost-benefit evalua
tion behavior: A) Schematic depicting the T-maze task with a cost-benefit conflict. Authors varied size of rewards on each arm of the T-maze. B) Rats’ preference for milk chocolate varying with concentration. C) Time spent under light exposure at low (7lux) versus high (2000 lux) intensity.
Compared to control, chronically stressed rats exhibited higher sensitivity to increases in chocolate milk concentration, and lower responsiveness to increased light intensity. As a result, chronically stressed rats were consistently attracted to the high concentrated milk (high reward) even when it was coupled with high intensity light (high cost), p<0.001 (one-way ANOVA with Bonferroni correction). Control rats were less inclined to choose high reward options if they were coupled with high costs, and therefore settled for options with lower concentrations of chocolate so long as they were coupled with low intensity light.
Evidence for changes in the prefrontal cortiostriatal circuit activity following 14 days of chronic stress.
Figure 3: Optogenetic excitement of the PFC-PL projections rescue decision-making behavior in chronically stressed rats.
A) Schematic of transfection of viral load onto PFC-PL neurons, and optogenetic control of synapses between PFC-PL and striosomal SPNs. B) Optogenetic excitation of PFC-PL during the Tmaze task. Optogenetic excitation of the PFC-PL neurons in chronically stressed rats lead them to make decisions that account for maximum value over absolute high risk-high reward. This means that, like control, the chronically stressed optogenetic mice settled for options with low intensity light over options with high intensity light coupled with high reward, p < 0.001 (one-way ANOVA with Bonferroni correction. This is considered to be adaptive behavior and indicative of successful costbenefit evaluation.
DISCUSSIONS AND CONCLUSIONS Prior to this study, it was known that chronic stress is a major contributor to the development of depression and anxiety, and that the impairment of value-based decision making is commonly experienced by individuals with depression or anxiety. Friedman and colleagues (2017) used behavioral and physiological measures to shed light on the direct link between chronic stress and decision making, and they concluded with compelling evidence to support their hypothesis. Firstly, it was found
that chronically stressed rats were unable to conduct correct cost-benefit evaluation, which lead to poor value-based decision-making. The stress-induced behavioral change also correlated with alterations in activity of the prefrontal corticostriatal circuitry known to be involved in decision-making. Following chronic stress, electrophysiological recording revealed reduced activity of this feed-forward inhibitory circuit, which lead to the increased excitation of striosomal projecting neurons. Uncoincidentally, these projections synapse with neurons of the substantia nigra that respond to motivating stimuli (rewarding and aversive) (Sousa & Almeida, 2012). The authors speculated that uncontrolled and excessive activity of the substantia nigra may be the mechanism through which chronic stress causes impairment in appropriate cost-benefit evaluation. This prediction was tested and supported when optogenetic excitation of the PFC-PL neurons rescued the decisionmaking behavior of chronically stressed mice. Overall, Friedman and colleagues’ (2017) study contributed greatly to our understanding of the intersection between environmental stressors, brain physiology and behavioral output. Until recently, the relationship between stress and decision making was mainly considered in the context of psychiatric disorders (Goschke, 2014; Stetz et al., 2007). This is because difficulties with decision making was often attributed to abnormal fluctuations in norepinephrine neurotransmission brought by stress-induced depression or anxiety (Goddard et al., 2010; Schildkraut, 1965). Although impairment in decision-making is often comorbid with such disorders, Friedman and colleagues’ (2017) study shows that the specialized circuit for value-based decision-making may be influenced by stress separate from other brain regions that control mood and anxiety, such as the anterior cingulate cortex, insula and amygdala (Pandya et al., 2012). This could explain why some individuals still suffer from stress induced decision-making impairment without necessarily experiencing depression or anxiety (Porcelli & Delgado, 2017). Likewise, Friedman et al.’s findings may have important clinical implications, since it supports the notion that stress is linked to a cluster of symptoms associated but not exclusive to psychiatric disorders.
CRITICAL ANALYSIS Although the cost-benefit conflict T-maze behavioral assay and methodology was described in Friedman et al. (2017)’s paper, the results from this assay was not depicted clearly by the authors. A brief description in the supplemental materials mentions that different combinations of concentration of chocolate milk were coupled with either high (2000lux) or low (7lux) light intensity, however, the authors did not present the ‘frequency of selection’ data for each combination of chocolate concentration and light intensity. Recording and presenting this data could give insight to trends in decision making, and reveal the threshold levels of costs and rewards where rats experience the most conflict. Likewise, the authors’ could be criticized. Bright fluorescent light is aversive for rats, and it is considered appropriate to apply as punishment in place of foot shocks (Barker et al., 2010). However, punishment is not synonymous with cost; for cost-benefit paradigms, cost is generally defined by the amount effort that is exerted to obtain a reward (Braun & Hauber, 2011; Walton et al., 2002). In contrast, punishment elicits negative affect and often causes distress, which could lead to confounding effects that influence judgement (Molm, 1994). Therefore, in the case of Friedman et al. (2017)’s experiment, chronically stressed rats may have behaved differently from control, not because of impaired costbenefit evaluation, but because of increased or decreased sensitivity to distressing punishment. Lastly, the authors suggested that ineffective feedforward inhibition in the prefrontal corticostriatal circuit is the cause for excessive activity of the substantia nigra neurons that control responses to rewarding and aversive stimuli. Although they quoted previous research to explain this line of logic, Friedman et al. (2017) did not carry out any physiological measures or optogenetic manipulations at the substantia nigra to confirm this. Hyperactivity in the striosomal projecting neurons may be influencing other brain regions besides the substantia nigra, and therefore decision-making impairment in this study cannot be confidently attributed to the substantia nigra, without evidence.
FUTURE DIRECTIONS To avoid confounding effects from distressing punishment with bright light, future studies continuing Freidman et al.’s (2017) work could implement a cost-benefit conflict T-maze task that uses barriers as cost instead of light. This setup was used in the past by Braun and Hauber (2011) and Walton et al. (2002), both of whom were interested in rodent brain function during decision-making. In both of these studies, the barriers were triangular shaped wooden blocks, and the rats had to climb up the vertical wall and climb down the steep slope to reach the reward on the other side. The cost was modified by increasing the height of the vertical wall of the barrier, from 15cm to 30cm in Walton et al.’s (2002) study, and from 20cm to 30cm in Braun and Hauber’s (2011) study. Braun and Hauber (2011) also manipulated reward by varying food reward size between full to half size. Considering experimental designs from previous literature, Friedman et al.’s study could implement block barriers of heights 15cm, 20cm, and 30cm, to provide 3 magnitudes of cost, and use 30%, 60% and 90% chocolate concentrations to provide 3 magnitudes of reward. This will make it simpler to match different sizes of costs and rewards, and it will ensure that the obtained results will not be influenced by confounding effects resulting from punishment. Furthermore, authors Friedman et al. could improve the transparency of their work by presenting their results clearly. For each trial, the cost-benefit options at the two arms of the TMaze should be reported, as well as the option that the rat chose. This will allow readers to follow the authors’ line of logic, and to derive more extensive interpretations from the data.
Similarly, the missing link between the substantia nigra and decision impairment must be addressed. Repeating the present experimental design and adding electrophysiological measures at the substantia nigra during the cost-benefit conflict T-maze task will be the first indicator of whether the prefrontal corticostriatal feed-forward inhibition increases activity in the subtantia nigra, as proposed by the authors. Friedman and colleagues’ (2017) study could be extended to expand our understanding of the relationship between chronic stress and addictive behaviors. Friedman et al. (2017)’s study found that chronically stressed rats gravitate to high-risk highreward alternatives, which is also the maladaptive behavior exhibited by individuals with gambling addictions (Fujimoto et al., 2017). Fujimoto and colleagues put forth evidence for reduced risk-attitude flexibility in participants with gambling disorders associated with reduced activity in the dlPFC. With this in mind, future studies could identify the influence of chronic stress on brain activity in the prefrontal corticostriatal circuit and the dlPFC while testing with the rodent model of the Iowa Gambling Task (IGT) (Van Den Bos et al., 2006). IGT is an experiment that tests behavioral responses to outcome uncertainty and payoffs between immediate and long-term gains. Applying this assay will increase the sophistication of Friedman et al.’s cost-benefit conflict paradigm by adding the dimension of future planning into decision making. According to Friedman et al. (2017), chronically stressed rats cannot evaluate costs and benefits as well as control, therefore stressed rats’ behavior is expected to reflect this as an increase impulsion in the IGT test, as well as a significant shift to short-term risky decisions that forgo low-risk/high-gain long-term options. Overall, implementing the IGT test as an extension to Friedman et al. (2017)’s study will not only expose the gravity of influence that chronic stress has on time-dependent value-decision making, but it will also have important implications for the interaction between chronic stress and addictive behaviors.
10. 11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21. Amemori, K. I., & Graybiel, A. M. (2012). Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nature Neuroscience, 15(5), 776–785. https://doi.org/10.1038/nn.3088 American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). https:// doi.org/10.1176/appi.books.9780890425596 Barker, D. J., Sanabria, F., Lasswell, A., Thrailkill, E. A., Pawlak, A. P., & Killeen, P. R. (2010). Brief light as a practical aversive stimulus for the albino rat. Behavioural Brain Research, 214(2), 402–408. https://doi.org/10.1016/j.bbr.2010.06.020 Braun, S., & Hauber, W. (2011). The dorsomedial striatum mediates flexible choice behavior in spatial tasks. Behavioural Brain Research, 220(2), 288–293. https://doi.org/10.1016/j.bbr.2011.02.008 Cousins, M. S., Atherton, A., Turner, L., & Salamone, J. D. (1996). Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behavioural Brain Research, 74(1–2), 189–197. https://doi.org/10.1016/0166-4328(95) 00151-4 Friedman, A., Homma, D., Gibb, L. G., Amemori, K. I., Rubin, S. J., Hood, A. S., Riad, M. H., & Graybiel, A. M. (2015). A corticostriatal path targeting striosomes controls decision-making under conflict. Cell, 161(6), 1320–1333. https://doi.org/10.1016/ j.cell.2015.04.049 Fujimoto, A., Tsurumi, K., Kawada, R., Murao, T., Takeuchi, H., Murai, T., & Takahashi, H. (2017). Deficit of state-dependent risk attitude modulation in gambling disorder. Translational Psychiatry, 7(4). https://doi.org/10.1038/tp.2017.55 Goddard, A. W., Ball, S. G., Martinez, J., Robinson, M. J., Yang, C. R., Russell, J. M., & Shekhar, A. (2010). Current perspectives of the roles of the central norepinephrine system in anxiety and depression. In Depression and Anxiety (Vol. 27, Issue 4, pp. 339–350). John Wiley & Sons, Ltd. https://doi.org/10.1002/da.20642 Goschke, T. (2014). Dysfunctions of decision-making and cognitive control as transdiagnostic mechanisms of mental disorders: Advances, gaps, and needs in current research. International Journal of Methods in Psychiatric Research, 23(S1), 41–57. https:// doi.org/10.1002/mpr.1410 Khan, S., & Khan, R. A. (2017). Chronic Stress Leads to Anxiety and Depression. Many Americans Stressed about Future of Our Nation, New APA Stress in America TM 2020, from https://www.apa.org/news/press/releases/2017/02/stressed-nation Survey Reveals. (n.d.). Retrieved June 9,
Molm, L. D. (1994). Is Punishment Effective? Coercive Strategies in Social Exchange. https://doi.org/10.2307/2786703 Social Psychology Quarterly, 57(2), 75.
Mukherjee, D., Lee, S., Kazinka, R., D Satterthwaite, T., & Kable, J. W. (2020). Multiple Facets of Value-Based Decision Making in Major Depressive Disorder. Scientific Reports, 10(1), 3415. https://doi.org/10.1038/s41598-020-60230-z Pandya, M., Altinay, M., Malone, D. A., & Anand, A. (2012). Where in the brain is depression? In Current Psychiatry Reports (Vol. 14, Issue 6, pp. 634–642). NIH Public Access. https://doi.org/10.1007/s11920-012-0322-7 Porcelli, A. J., & Delgado, M. R. (2017). Stress and decision making: effects on valuation, learning, and risk-taking. In Current Opinion in Behavioral Sciences (Vol. 14, pp. 33–39). Elsevier Ltd. https://doi.org/10.1016/j.cobeha.2016.11.015 Schildkraut, J. J. (1965). The catecholamine hypothesis of affective disorders: a review of supporting evidence. In The American journal of psychiatry (Vol. 122, Issue 5, pp. 509–522). American Psychiatric Publishing. https://doi.org/10.1176/ajp.122.5.509 Schweimer, J., & Hauber, W. (2006). Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learning and Memory, 13(6), 777–782. https://doi.org/10.1101/lm.409306 Sousa, N., & Almeida, O. F. X. (2012). Disconnection and reconnection: The morphological basis of (mal)adaptation to stress. In Trends in Neurosciences (Vol. 35, Issue 12, pp. 742–751). Elsevier Current Trends. https://doi.org/10.1016/j.tins.2012.08.006 Stetz, M. C., Thomas, M. L., Russo, M. B., Stetz, T. A., Wildzunas, R. M., McDonald, J. J., Wiederhold, B. K., & Romano, J. A. (n.d.). Stress, Mental Health, and Cognition: A Brief Review of Relationships and Countermeasures. Van Den Bos, R., Lasthuis, W., Den Heijer, E., Van Der Harst, J., & Spruijt, B. (2006). Toward a rodent model of the Iowa gambling task. Behavior Research Methods, 38(3), 470–478. https://doi.org/10.3758/BF03192801 Walton, M. E., Bannerman, D. M., & Rushworth, M. F. S. (2002). The role of rat medial frontal cortex in effort-based decision making. Journal of Neuroscience, 22(24), 10996–11003. https://doi.org/10.1523/JNEUROSCI.22-24-10996.2002










