
16 minute read
Role of Lateral Hypothalamic Dopaminergic Mechanisms in Feeding Regulation: A Study
Caitlin Therence Tejowinoto
The mesolimbic dopaminergic pathway, also known as the reward pathway, has been associated with motivation of certain behaviours in living organisms. Food intake is one of the key components of survival and studies have shown that food consumption results in dopamine release in the reward pathway, suggesting that dopaminergic mechanisms may be important in feeding regulation. The lateral hypothalamus is also thought to be a key player in feeding regulation because it has been linked to motivation of food consumption, innervated by appetite-affecting neurons from the arcuate nucleus, and the presence of neuropeptides that affects feeding behaviour such as melanocortin concentrating hormone and orexin. Presence of dopamine receptors in the lateral hypothalamus has been reported, hence Yonemochi et al. (2019) investigated the role of dopaminergic mechanisms in the lateral hypothalamus in regard to feeding regulation. It was found that the dopaminergic function in the lateral hypothalamus regulates feeding by a negative feedback mechanism, where the release of dopamine from feeding stimulates dopamine receptors in the lateral hypothalamus, which reduces food intake. This study is important in investigating the homeostasis of food intake and energy balance, which can be applied to energy balance disorders.
Advertisement
Keywords: Dopamine, feeding regulation, lateral hypothalamus, neuropeptides, food intake, dopamine receptors
Introduction
Feeding is essential as it maintains energy balance, allowing organisms to perform basic bodily functions. Chronic disruptions of energy balance can lead to energy balance disorders like obesity and anorexia, which can be detrimental to one’s health (Reynolds et al., 2015). Dopaminergic pathways in the central nervous system is a significant component of the brain’s reward system. The reward system motivates organisms into the LH of rats, which were then left for a week. Following
to perform certain behaviours such as feeding, drinking, and reproduction (Arias-Carrión et al., 2010). Studies have shown that feeding, especially the consumption of palatable food, results in a release of dopamine in the mesolimbic dopaminergic pathway (Hajnal & Norgen, 2001; Schwartz et al., 2010; Yonemochi et al., 2019). Therefore, the mesolimbic dopaminergic pathway is thought to play a key role in regulating feeding behaviour, specifically promoting the intake of preferred food. Another key to survival is homeostasis, defined as the process of maintaining a stable internal environment which is mainly regulated by the hypothalamus. A specific region within the hypothalamus, the arcuate nucleus (ARC), is responsible for feeding and metabolism regulation. ARC contains two types of neurons: 1) the agouti-related peptide (AgRP) and neuropeptide Y (NPY) expressing neurons (AgRP/NPY) which are orexigenic, meaning that they increase appetite, and 2) the proopiomelanocortin (POMC) expressing neurons (POMC) which are anorexigenic, which suppresses appetite (Timper & Brüning, 2017). These neurons project to the lateral hypothalamus (LH), where more neuropeptides that affect feeding behaviour such as melanocortin concentrating hormone (MCH) and orexin are found, thus, LH is suggested to contribute to feeding regulation as well (Sakurai et al., 1998; Brown et al., 2015; Yonemochi et al., 2019). More reasons that LH may be important in feeding is that lesion of LH in rats caused a loss of motivation to eat or drink even though the rats were still physically able to do so, resulting in starvation and dehydration; and electrical stimulation of LH can motivate feeding (Brown et al., 2015; Stuber & Wise, 2016). Dopamine levels in the LH were found to be correlated be found within the LH (Meguid et al., 1995; Fetissov et al., 2002; Ikeda et al., 2018). A study by Ikeda et al. (2018) found that inhibition of dopamine receptors in LH increases food intake in mice, suggesting the possibility of a negative feedback mechanism through these dopamine receptors. Hence, dopaminergic function within the LH is suggested to play a key role in feeding behaviour regulation. take?; 4) What are the roles of dopamine receptors D1 and D2 within the LH on neuropeptides (Orexin, MCH, NPY, AgRP, POMC)? Answering these questions would clarify whether dopaminergic mechanisms in LH regulates feeding through hypothalamic neuropeptides.
Methods
To analyze the relationship between food intake, glucose, and LH dopamine, mice were fasted for 16 hours, then either re-fed or injected by glucose (control mice were fasted throughout the process) while monitoring the LH dopamine levels. Dopamine levels were monitored using in-vivo microdialysis; dialysates were collected every 20 minutes pre- and post - refeeding. Dopaminergic neuron projections were visualized using the retrograde tracer Fluoro-gold (FG). FG were injected with food intake and dopamine receptors have been shown to
the week, the brains were removed, fixed with paraformaldehyde, and then sectioned. Sections were also incubated with an antibody against tyrosine hydroxylase (TH), an enzyme responsible for the synthesis of dopamine. To investigate the role of D1 and D2 receptors, mice were fasted for 16 hours. Following fasting, mice were injected with either D1 agonist (SKF 38393 hydrochloride), D1 antagonist (SCH 23390 hydrochloride), D2 agonist (quinpirole hydrochloride), or D2 antagonist (lsulpiride) and their cumulative food intake was monitored up to 4 hours following injection. Motor impairments due to dopamine deficiency can affect feeding behaviour, hence drugs were administered at a dose that mice locomotor activity is unaffected (Schwartz et al., 2010). Reverse transcription was also done to quantify the amount of mRNA of the neuropeptides of interest. An hour following drug injections, mice hypothalamus were dissected and the RNA were isolated. Reverse transcription was conducted followed by PCR, then analyzed using electrophoresis.
Major Results
Food Intake, Glucose, and LH Dopamine In a fasted state, LH dopamine levels remained relatively steady. Food intake and glucose injection were both found to increase LH dopamine levels (Fig. 1). This is consistent with previous findings by Meguid et al. (1995), where they found that dopamine release in LH is correlated with meal size. Yonemochi et al. (2019) suggests that food intake increases blood glucose levels, which then stimulates glucose-responsive neurons in the hypothalamus.
Yonemochi et al. (2019)’s Study
Yonemochi et al. (2019) followed up on Ikeda et al. (2018)’s findings by conducting a study investigating the role of dopaminergic mechanisms in LH on feeding. The study aimed to answer the following questions: 1) What are the effects of food intake and glucose on LH dopamine levels?; 2) How do dopaminergic neurons project to the LH?; 3) What are the roles of dopamine receptors D1 and D2 within the LH on food in

Fig. 1. Adapted from Yonemochi et al. (2019). Effect of refeeding (left) and glucose injection (right) on LH dopamine levels. Both refeeding and glucose injection increases LH dopamine levels.
Projections of Dopaminergic Neurons to LH FG were found to be present in the VTA and SNc, indicating that the neurons projected from VTA and SNc to LH. TH were also found in most FG-positive cells. Since TH is a dopamine precursor, the presence of TH confirms that the neurons were indeed dopaminergic neurons. rats resulted in a decrease of daily food intake and body weight. It has also been previously reported that striatal D2 receptors were downregulated in obese rats and knocking down of D2 resulted in compulsive feeding (Johnson & Kenny, 2010). These findings suggest that D1 and D2 activation terminates feeding. However, a study by Chen et al. (2014) found that injection of a different D1 agonist, SKF81297, into the perifornical LH led to an increase in food and alcohol intake, which contradicts the findings of the present study. A possible reason for this discrepancy is the possibility of different agonists giving rise to different effects, thus, additional research in this area is still needed.
Role of D1 and D2 Receptors on Food Intake When mice were injected with D1 receptor agonist (SKF 38393) or D2 receptor agonist (quinpirole), cumulative food intake is lower than control (Fig. 2). When the receptors are blocked by the antagonists, no change in food intake was observed. However, when the receptor agonists were administered together with the antagonists, there is no overall effect on food intake. Role of D1 and D2 Receptors on Hypothalamic Neuropeptides Stimulation of D1 receptors did not affect the mRNA of preproorexin nor MCH, while stimulation of D2 receptors decreased the amount of preproorexin mRNA but did not affect mRNA of MCH (Fig. 3). It has been discovered that the activity of orexin neurons decreases during refeeding or high blood glucose levels (Brown et al., 2015). This agrees with the present study’s findings that suggests feeding increases LH dopamine levels, which stimulates D2 receptors, which in turn inhibits the activity of orexin neurons hence the low preproorexin levels.
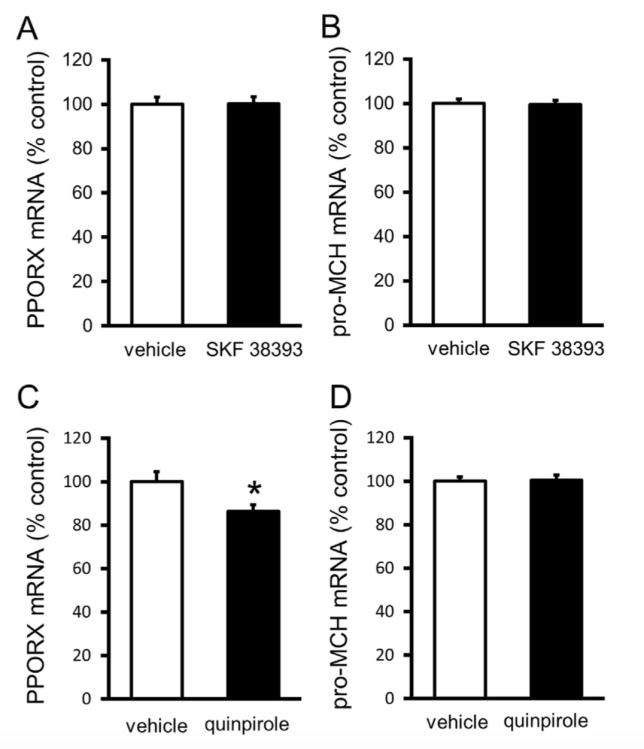

Fig. 3. Adapted from Yonemochi et al. (2019). Effect of activation of dopamine receptors on preproorexin mRNA (left) and pro-MCH mRNA (right). A and B represents activation of D1 receptors, C and D represents activation of D2 receptors. Activation of dopamine D2 receptors (by administration of quiniprole) decreases preproorexin mRNA.
Fig.2. Adapted from Yonemochi et al. (2019). Cumulative food intake following manipulation of lateral hypothalamic D1 receptors (left) and D2 receptors (right). Stimulation of D1 and D2 receptors result in decreased food intake.
Same results were found in a study done by Kuo (2002), where co-administration of SKF 38393 and quinpirole in Although not shown in a figure, Stimulation of D1 receptors decreased the mRNA levels of NPY and AgRP, while stimulation of D2 receptors increased the mRNA levels of POMC. These results suggest a possibility that the stimulation of D1 receptors decreases feeding by inhibiting NPY/AgRP neurons, while D2 receptors in the LH decreases feeding by inhibiting orexin neurons and stimulating POMC neurons. This present study did not uncover the mechanisms behind the observed phenomena. Yonemochi et al. (2019) suspected that LH
D1 and D2 receptors affect NPY/AgRP and POMC neurons through GABA signalling. This idea is supported by the findings by de Vrind et al. (2019), where the activation of LH GABA neurons that coexpress the leptin receptor resulted in a decrease in body weight and food intake. However, the mechanism is still unclear.
Conclusions
The present study by Yonemochi et al. (2019) found that: 1) food intake increases dopamine levels in the LH, 2) dopaminergic neurons project from the VTA and SNc to the LH, 3) stimulation of dopamine D1 and D2 receptors in the LH deThe present study discovered that stimulation of D1 stimulates POMC neurons respectively, however, the underly
creases food intake, and 4) stimulation of D1 receptors in LH studies have the potential to be developed further to investi
inhibits NPY/AgRP neurons while stimulation of D2 receptors inhibits orexin neurons and stimulates POMC neurons. The authors came to a conclusion that feeding stimulates dopaminergic neurons that project to the LH from VTA and SNc, which acts as a negative feedback to terminate food intake through dopamine D1 and D2 receptors, possibly through neuropeptides.
The LH is involved in both motivation and homeostatic pathways. It has been previously thought that hypothalamic dopamine signalling through neurons in dorsomedial and ARC inhibits food intake (Schwartz et al., 2000). Although the present study did not establish a detailed pathway, it confirms that the dopaminergic pathway has inhibitory effects on food inwhich are responsible for the regulation of feeding behaviour.
take. The findings serve as a good starting point for future investigation of the mechanisms behind this phenomena. This regulation of feeding behaviour in the LH maintains the body’s energy balance. Looking into the underlying mechanism is important to further understand energy balance disorders and how to restore energy balance through either new medication or therapy methods.
Critical Analysis
The present study by Yonemochi et al. (2019) clarified the role of dopaminergic function in the LH in the regulation of feeding. The results of the present study mostly agree with published literature, except for the study by Chen et al. (2014) on the role of D1 and D2 in LH on food and alcohol intake, where they found that stimulation of D1 promotes food and alcohol consumption, whereas stimulation of D2 suppresses alcohol consumption. The present study found that both D1 and D2 suppress feeding behaviour when stimulated. Some possible reasons for this discrepancy are the usage of a different D1 agonist (SKF81297) by Chen et al. (2014), or that Chen et al. (2014) studied a specific part of the LH, the perifornical lateral hypothalamus (PF/LH) which may have different effects on feeding compared to LH as a whole. The neuropeptides studied in this present study are: NPY, AgRP, orexin, and POMC. Other neuropeptides that the rotensin is thought to also play a role in the regulation of feeding, as central Nts has been shown to reduce feeding in rodents. Nts neurons are activated by leptin, and the lateral hypothalamic area is the only site where leptin receptor (LepR) co -expressing Nts neurons are present. These neurons, along with the regular Nts neurons present in the LH, project to the VTA, suggesting that leptin and Nts may play a role in feeding regulation through dopaminergic mechanisms. and D2 receptors in the LH inhibits NPY/AgRP neurons and ing mechanisms are yet to be thoroughly investigated. Following the findings of this study, the researchers should uncover the precise mechanism on how D1 and D2 receptors affect NPY/AgRP and POMC neurons. Another thing to note is that Yonemochi et al. (2019)’s study was conducted on male ICR mice and male Wistar rats. While sex was kept the same for the sake of consistency and reducing variability, the researchers should have included one more (separate) group of female mice and rats. Male biology and female biology are different, hence the effects seen in male models might not be observed in female models. These gate energy balance homeostasis and treatment for energy balance disorders. Female representation in these studies are greatly needed so that an effective treatment can be achieved for everyone.
Future directions
How stimulation of D1 and D2 receptors in the LH inhibits NPY/AgRP neurons and stimulates POMC neurons is still unclear. Yonemochi et al. (2019) proposed a possible mechanism underlying these phenomena through GABA interneurons found in the LH (Fig. 4). This suggested experiment will clarify how the dopamine D1 and D2 receptors affect neuropeptides authors can investigate are neurotensin (Nts) and leptin. Neu

Fig. 4. Yonemochi et al. (2019)’s proposed mechanism of how LH D1 and D2 receptors affect NPY/AgRP neurons and POMC neurons through activation and inhibition of GABA.
LH D1 Receptors’ Effect on NPY/AgRP Neurons Experiments will be done on D2R-knockout mice. Mice will be separated into 3 treatments: 1) cerebral injection of D1
agonist; 2) cerebral injection of D1 agonist and GABA antagonist, and 3) control. Procedure will be similar to the present study by Yonemochi et al. (2019). After drug injection, the mice hypothalamus will be dissected, followed by RNA isolation, then reverse transcription. The mRNA present in the samples will be analyzed using electrophoresis. The mice group injected with D1 agonist only would have decreased levels of NPY and AgRP mRNA compared to the control group. If the suggested mechanism in Fig. 4 were to be true, when GABA antagonist is injected along with D1 agonist, there will be no decrease in NPY and AgRP mRNA as GABA interneurons will not inhibit NPY/AgRP neurons.
LH D2 Receptors’ Effect on POMC Neurons To investigate the effect of D2 receptors, experiment will be done on D1R-knockout mice. Mice will be separated into 3 groups: 1) cerebral injection of D2 agonist; 2) cerebral injection of D2 agonist and GABA agonist, and 3) control. Just like mentioned above, mice will undergo drug injection, hypothalamus dissection, RNA isolation, reverse transcription, and electrophoresis. Injection of D2 agonist will result in an increase of POMC mRNA compared to the control. If the suggested pathway in Fig. 4 were to be true, when GABA agonist is injected along with the D2 agonist, there will be no increase in POMC as the POMC neurons are inhibited by the GABA agonists.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17. Arias-Carrión, O., Stamelou, M., Murillo-Rodríguez, E., Menéndez-González, M., & Pöppel, E. (2010). Dopaminergic reward system: A short integrative review. International Archives of Medicine, 3(1), 24. https://doi.org/10.1186/1755-7682-3-24 Brown, J. A., Woodworth, H. L., & Leinninger, G. M. (2015). To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Frontiers in Systems Neuroscience, 9. https://doi.org/10.3389/ fnsys.2015.00009 Chen, Y.-W., Morganstern, I., Barson, J. R., Hoebel, B. G., & Sarah F. Leibowitz. (2014). Differential Role of D1 and D2 Receptors in the Perifornical Lateral Hypothalamus in Controlling Ethanol Drinking and Food Intake: Possible Interaction with Local Orexin Neurons. Alcoholism: Clinical and Experimental Research, 38(3), 777–786. https://doi.org/10.1111/ acer.12313 de Vrind, V. A. J., Rozeboom, A., Wolterink‐Donselaar, I. G., Luijendijk‐Berg, M. C. M., & Adan, R. A. H. (2019). Effects of GABA and Leptin Receptor‐Expressing Neurons in the Lateral Hypothalamus on Feeding, Locomotion, and Thermogenesis. Obesity, oby.22495. https://doi.org/10.1002/oby.22495 Dong-Yih Kuo. (2002). Co-Administration of Dopamine D1 and D2 Agonists Additively Decreases Daily Food Intake, Body Weight and Hypothalamic Neuropeptide Y Level in Rats. Journal of Biomedical Science, 9(2), 126–132. https:// doi.org/10.1007/bf02256023 Fetissov, S. O., Meguid, M. M., Sato, T., & Zhang, L.-H. (2002). Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 283(4), R905–R910. https://doi.org/10.1152/ajpregu.00092.2002 Hajnal, A., & Norgren, R. (2001). Accumbens dopamine mechanisms in sucrose intake. Brain Research, 904(1), 76–84. https://doi.org/10.1016/S0006-8993(01)02451-9 Ikeda, H., Yonemochi, N., Ardianto, C., Yang, L., & Kamei, J. (2018). Pregabalin increases food intake through dopaminergic systems in the hypothalamus. Brain Research, 1701, 219–226. https://doi.org/10.1016/j.brainres.2018.09.026 Johnson, P. M., & Kenny, P. J. (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience, 13(5), 635–641. https://doi.org/10.1038/nn.2519 Meguid, M. M., Yang, Z.-J., & Koseki, M. (1995). Eating induced rise in LHA-dopamine correlates with meal size in normal and bulbectomized rats. Brain Research Bulletin, 36(5), 487–490. https://doi.org/10.1016/0361-9230(95)92128-3 O’Connor, E. C., Kremer, Y., Lefort, S., Harada, M., Pascoli, V., Rohner, C., & Lüscher, C. (2015). Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron, 88(3), 553–564. https://doi.org/10.1016/ j.neuron.2015.09.038 Reynolds, C., Gray, C., Li, M., Segovia, S., & Vickers, M. (2015). Early Life Nutrition and Energy Balance Disorders in Offspring in Later Life. Nutrients, 7(9), 8090–8111. https://doi.org/10.3390/nu7095384 Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., Williams, S. C., Richardson, J. A., Kozlowski, G. P., Wilson, S., Arch, J. R. S., Buckingham, R. E., Haynes, A. C., Carr, S. A., Annan, R. S., McNulty, D. E., Liu, W.-S., Terrett, J. A., Elshourbagy, N. A., … Yanagisawa, M. (1998). Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior. Cell, 92(4), 573–585. https://doi.org/10.1016/S0092- 8674(00)80949-6 Schwartz, M. W., Woods, S. C., Porte, D., Seeley, R. J., & Baskin, D. G. (2000). Central nervous system control of food intake. Nature, 404(6778), 661–671. https://doi.org/10.1038/35007534 Stuber, G. D., & Wise, R. A. (2016). Lateral hypothalamic circuits for feeding and reward. Nature Neuroscience, 19(2), 198–205. https://doi.org/10.1038/nn.4220 Timper, K., & Brüning, J. C. (2017). Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Disease Models & Mechanisms, 10(6), 679–689. https://doi.org/10.1242/dmm.026609 Yonemochi, N., Ardianto, Chrismawan, Yang, Lizhe, Yamamoto, Shogo, Ueda, Daiki, Kamei, Junzo, Waddington, John L., & Ikeda, Hiroko. (2019). Dopaminergic mechanisms in the lateral hypothalamus regulate feeding behavior in association with neuropeptides. Biochemical and Biophysical Research Communications, 6.










