
12 minute read
Curcumin as a Potential Treatment in Parkinson’s Disease
Cathy Xiong
Parkinson’s disease (PD) is a progressive neurodegenerative condition characterized by the misfolded alpha-synuclein proteins, the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), along with motor deficits and nonmotor symptoms (Balestrino & Schapira, 2020; Klingelhoefer & Reichmann, 2015). There is currently no cure for PD and treatments mainly use dopamine substitutes or deep brain stimulation to treat the motor symptoms (Connolly & Lang, 2014; Okun, 2012). Many studies were investigating potential therapeutic uses of the natural substances derived from plants (Scapagnini et al., 2011). Curcumin, a common ingredient in curry as turmeric, is known to be an antioxidizing and anti-inflammatory agent (Sharma, Gescher, & Steward, 2005). The current study by Khatri and Juvekar (2016) administrated rotenone in mice to produce the Parkinsonian symptoms and tried to combat it with curcumin. The study aimed to investigate the efficacy of curcumin on reversing the damage done by rotenone in mice using behavioural and biochemical examinations. The rotenone treated mice performed poorly on the rotarod test and were less mobile in the actophotometer and open field test compared to the control mice. The administration of rotenone also increased the levels of lipid peroxidation and nitrite, and activity of acetylcholinesterase, and decreased the activities of antioxidant enzymes, and mitochondrial complex enzymes. Results revealed that curcumin treated mice restored the negative effect of rotenone on both motor and cognitive functions and improved the impairment on oxidative defence and enzyme activities in mitochondrial complexes.
Advertisement
Keywords: Parkinson’s disease (PD), rotenone, curcumin, motor deficits, antioxidant, mitochondrial complex, mice model
mental groups got the same rotenone injection with curcumin (Cur) oral treatment of 50 mg/kg, 100 mg/kg, or 200 mg/kg, respectively. Before the experiment started, the mice had a week to adapt to the new environment, and then all mice had practiced on the rotarod apparatus for another five days. An initial behavioural test was done and set as day zero. On day one, the mice were treated as mentioned above with the behavioural examination. After repeated for twenty-one times, all mice were sacrificed on day twenty-two for measures in brain tissue.
Major Results Behavioural Observations
Figure 1. Visual abstract for the outline of the experiment performed by Khatri and Juvekar (2016) There was no difference in all groups for the actophotometer test on day 0. On day 21, ROT treated group showed a signifiFor Cur treated groups, as the dosage of curcumin increased,
Background and Introduction
As the second most common neurodegenerative disease after Alzheimer’s, 10% - 15 % of the Parkinson’s disease (PD) can be attributed to genetics while the rest are due to unknown factors and considered environmental (Emamzadeh & Surguchov, 2018). The known pathology of PD is the accumulation and aggregation of the misfolded proteins called alpha-synuclein into Lewy bodies, which is a hallmark of PD (Balestrino & Schapira, 2020). Oligomers formed by α-synucleins are toxic to the cells and cause the dopaminergic neural death in the substantia nigra pars compacta(SNpc), which results in the disturbances in motor functions (Ciechanover & Kwon, 2015). Patients with PD display slow and rigid movements, resting tremors, and impairments in gait and posture, which precede by nonmotor symptoms (Klingelhoefer & Reichmann, 2015). Furthermore, the significant reduction of mitochondrial complex I is another key feature in PD, and is thought to associated with
cant decrease in locomotion compared to the control group. dementia in PD (Gatt et al., 2016).
the more locomotive activity was performed, compared to ROT treated group. On the rotarod test, a similar trend was observed as the actophotometer test, where a significant reduction of time remaining on the rotarod was found in ROT treated group compared to the control, and gradual increases were found in Cur treated groups compared to ROT. In the open field test (OFT), line crossing, grooming, and rearing followed the same trend. ROT treated mice showed longer immobility time compared to all other mice on day 21.

Spices and herbs have always been an essential to both the culinary and pharmacological aspects of the human history. Apart from their contribution to the colouring and flavouring of food, their polyphenolic properties also benefits the brain in preventing conditions associate with age, such as cancer and neurodegenerative diseases (Scapagnini et al., 2011). Curcumin motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson's disease”, by D. K. Khatri, & A. R.
has been used in ancient herbal medicine for its antioxidant, anti-inflammatory, and neuroprotective effects (Witkin & Li, 2013). Study has found curcumin to attenuate cytotoxicity and inhibit apoptosis (Fan et al., 2017).
The current study by Khatri and Juvekar (2016) used curcumin to test its ability against rotenone-induced neuronal death and movement disabilities. Thirty Swiss albino male mice were randomly assigned into 5 groups, including the control group, the negative control group, and the treatment groups. The control were injected with rotenone (ROT) 1 mg/kg. The three experiFigure 2. Behavioural observations for day 0 and day 21 in OFT on (A) line crossing, (B) rearings, (C) grooming, and (D) immobility time in all groups. Adapted from “Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced
group received vehicle control, and the negative control group Juvekar, Pharmacology Biochemistry and Behavior, 150–151, 39–47.
Biochemical Analysis
the ROT treated group compared to control and Cur treated groups showed a gradual decrease compared to ROT treated group. The similar pattern was also found in acetylcholinesterase (AChE) activity. For activities of superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH), ROT treated group showed a significant decrease compared to control and Cur increased the activities compared to ROT. The succinate dehydrogenase (SDH) and MTT reduction activities displayed a similar trend.
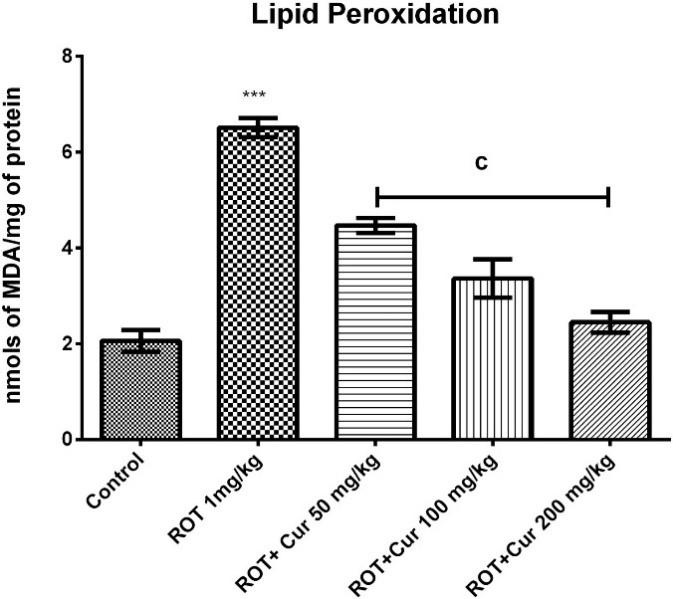

Figure 3. Levels of LPO and SDH activity in all groups. Adapted of oxidation and mitochondrial impairment. Therefore, the reduce their toxicity both in vitro and in vivo, which suggested that curcumin are relevant in modulating, or even halting, the α -synuclein aggregation in PD (Singh et al., 2013). A similar finding was also revealed by another study, where Gautam, Karmakar, Bose, & Chowdhury (2014) found that a mixture of curcumin and β-cyclodextrin could suppress protein aggregation, and even disaggregate the ones that already formed, making it a potential treatment in preventing PD. As one of the neurodegenerative diseases, PD also has features such as dementia and protein aggregation like Alzheimer’s and Huntington’s (Ciechanover & Kwon, 2015; Gatt et al., 2016). Therefore, if curcumin can help with PD, it might also be beneficial in other
from “Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson's disease”, by D. K. Khatri, & A. R. Juvekar, Pharmacology Biochemistry and coordination due to paralysis of the limbs of the mice, which confirmed the Parkinsonian damage done by rotenone (Khatri a test examining the dopaminergic neurons or the dopamine
Behavior, 150–151, 39–47.
Conclusion and Discussion
The results revealed the neuroprotective role of curcumin on the brain of the rotenone-induced Parkinsonian mice. The behavioural observations illustrated that long-term administration of rotenone resulted in reduced overall behavioural activity by actophotometer, impaired motor coordination by rotarod, and longer immobility duration in the OFT(Khatri & Juvekar, 2016). Analysis showed that rotenone increased the level of LPO and nitrite, which meant there was higher oxidative stress in rotenone treated mice (Khatri & Juvekar, 2016). Another increase in AChE level was shown in ROT treated mice, which indicated a decrease in acetylcholine at synapse and therefore reduced cognitive functions (Khatri & Juvekar, 2016). On the other hand, Cur treated mice showed higher levels of SOD, CAT, and GSH compared to ROT treated mice, which revealed the antioxidant ability of curcumin (Khatri & Juvekar, 2016). ROT treated mice showed a decrease in both SDH and curcumin helped improved (Khatri & Juvekar, 2016). These findings are consistent with the study done by Ramsés García-Niño & Pedraza-Chaverrí (2014), which found that curcumin maintained the levels of SOD, CAT, and GSH, and protect against mitochondrial alterations under the oxidative stress of heavy metals. findings suggested that curcumin has the potential to be used therapeutically in managing PD (Khatri & Juvekar, 2016). This is an important finding because PD are mostly due to unknown causes and is incurable, and current pharmacological treatments and deep brain stimulation are targeting at reducing motor symptoms. As the result of Khatri and Juvekar (2016) has shown, curcumin not only can improve the behavioural symptoms, but also on the biochemical level. Furthermore, a study discovered that curcumin can bind to oligomers and fibrils and neurodegenerative diseases.
Critical Analysis
The behavioural results showed impairment in locomotion and & Juvekar, 2016). However, the current paper did not perform MTT reduction that led to mitochondrial dysfunction, which
level, which is the reason for the motor deficits. Therefore, maybe the authors can include the influences of any effects of rotenone and curcumin on dopamine in the brain in order to make the experiment more closely relevant to PD. Also, the authors in the current paper mentioned no limitations about the experiment. As an oral treatment, the authors should mention the bioavailability of curcumin. Curcumin is poorly soluble in water and highly unstable in the digestive tract, making its protein disaggregating task still challenging in vivo (Gautam et al., 2014). Study using even a high dose of curcumin (12g/day) in human showed a weak bioavailability due to its low absorption and quick metabolizing and eliminating from the body (Anand, Kunnumakkara, Newman, & Aggarwal, 2007).
Future Directions
Future studies should focus on developing curcumin formulas with enhanced bioavailability for using in PD or other neurodegenerative diseases. Ways like pairing curcumin with other agents that increase its bioavailability, modifying its structure, or finding a derivative that works better in human body should be studied. Other spices or herbal medicines can be used with curcumin for a synergic effect that boost the efficacy, such as crocin that manages cell death, loganin that decreases neuroinflammation, and icariin that protects mitochondria function
(Chen et al., 2020). There has been study that used piperine from black pepper, which inhibits drug elimination at intestines, to increase the bioavailability of curcumin by 2000% in both rats and humans (Shoba et al., 1998). In the same setting as the current paper, mice can be group into control, negative control, and mice with the three combinations of curcumin and crocin, loganin, and icariin. The combination of better results can be delivered using intranasal administration for better uptake by the brain tissue (Prasad, Tyagi, & Aggarwal, 2014). Furthermore, several studies had used the nanoparticle approach as a deliver method to overcome the low bioavailability of curcumin (Ipar, Dsouza, & Devarajan, 2019; Umerska et al., 2018; Xie et al., 2011). Overall, more investigations can be done in improving the efficacy and bioavailability of curcumin by trying out different agents and diliveries.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13. Anand, P., Kunnumakkara, A. B., Newman, R. A., & Aggarwal, B. B. (2007). Bioavailability of curcumin: Problems and promises. Molecular Pharmaceutics. https://doi.org/10.1021/mp700113r
Balestrino, R., & Schapira, A. H. V. (2020, January 27). Parkinson disease. European Journal of Neurology. Blackwell Publishing Ltd. https://doi.org/10.1111/ene.14108
Chen, S. Y., Gao, Y., Sun, J. Y., Meng, X. L., Yang, D., Fan, L. H., … Wang, P. (2020, April 22). Traditional Chinese Medicine: Role in Reducing β-Amyloid, Apoptosis, Autophagy, Neuroinflammation, Oxidative Stress, and Mitochondrial Dysfunction of Alzheimer’s Disease. Frontiers in Pharmacology. Frontiers Media S.A. https://doi.org/10.3389/fphar.2020.00497
Ciechanover, A., & Kwon, Y. T. a. (2015, March 13). Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Experimental & Molecular Medicine. Nature Publishing Group. https:// doi.org/10.1038/emm.2014.117
Connolly, B. S., & Lang, A. E. (2014, April 23). Pharmacological treatment of Parkinson disease: A review. JAMA - Journal of the American Medical Association. American Medical Association. https://doi.org/10.1001/jama.2014.3654
Emamzadeh, F. N., & Surguchov, A. (2018, August 30). Parkinson’s disease: Biomarkers, treatment, and risk factors. Frontiers in Neuroscience. Frontiers Media S.A. https://doi.org/10.3389/fnins.2018.00612
Fan, C. dong, Li, Y., Fu, X. ting, Wu, Q. jian, Hou, Y. jun, Yang, M. feng, … Sun, B. liang. (2017). Reversal of Beta-AmyloidInduced Neurotoxicity in PC12 Cells by Curcumin, the Important Role of ROS-Mediated Signaling and ERK Pathway. Cellular and Molecular Neurobiology, 37(2), 211–222. https://doi.org/10.1007/s10571-016-0362-3
García-Niño, W. R., & Pedraza-Chaverrí, J. (2014). Protective effect of curcumin against heavy metals-induced liver damage. Food and Chemical Toxicology. https://doi.org/10.1016/j.fct.2014.04.016
Gatt, A. P., Duncan, O. F., Attems, J., Francis, P. T., Ballard, C. G., & Bateman, J. M. (2016). Dementia in Parkinson’s disease is associated with enhanced mitochondrial complex I deficiency. Movement Disorders, 31(3), 352–359. https:// doi.org/10.1002/mds.26513
Gautam, S., Karmakar, S., Bose, A., & Chowdhury, P. K. (2014). β-cyclodextrin and curcumin, a potent cocktail for disaggregating and/or inhibiting amyloids: A case study with α-synuclein. Biochemistry, 53(25), 4081–4083. https:// doi.org/10.1021/bi500642f
Ipar, V. S., Dsouza, A., & Devarajan, P. V. (2019). Enhancing Curcumin Oral Bioavailability Through Nanoformulations. European Journal of Drug Metabolism and Pharmacokinetics. https://doi.org/10.1007/s13318-019-00545-z
Khatri, D. K., & Juvekar, A. R. (2016). Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson’s disease. Pharmacology Biochemistry and Behavior, 150–151, 39–47. https://doi.org/10.1016/j.pbb.2016.09.002
Klingelhoefer, L., & Reichmann, H. (2015). Pathogenesis of Parkinson disease - The gut-brain axis and environmental factors. Nature Reviews Neurology. https://doi.org/10.1038/nrneurol.2015.197
15.
16.
17.
18.
19.
20.
21.
22. Okun, M. S. (2012, October 18). Deep-brain stimulation for Parkinson’s disease. New England Journal of Medicine. Massachussetts Medical Society. https://doi.org/10.1056/NEJMct1208070
Prasad, S., Tyagi, A. K., & Aggarwal, B. B. (2014). Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Research and Treatment. Korean Cancer Association. https://doi.org/10.4143/crt.2014.46.1.2
Scapagnini, G., Sonya, V., Nader, A. G., Calogero, C., Zella, D., & Fabio, G. (2011). Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Molecular Neurobiology, 44(2), 192–201. https://doi.org/10.1007/s12035-011-8181-5
Sharma, R. A., Gescher, A. J., & Steward, W. P. (2005). Curcumin: The story so far. European Journal of Cancer, 41(13), 1955–1968. https://doi.org/10.1016/j.ejca.2005.05.009
Shoba, G., Joy, D., Joseph, T., Majeed, M., Rajendran, R., & Srinivas, P. S. S. R. (1998). Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica, 64(4), 353–356. https://doi.org/10.1055/s-2006- 957450
Singh, P. K., Kotia, V., Ghosh, D., Mohite, G. M., Kumar, A., & Maji, S. K. (2013). Curcumin modulates α-synuclein aggregation and toxicity. ACS Chemical Neuroscience, 4(3), 393–407. https://doi.org/10.1021/cn3001203
Umerska, A., Gaucher, C., Oyarzun-Ampuero, F., Fries-Raeth, I., Colin, F., Villamizar-Sarmiento, M. G., … Sapin-Minet, A. (2018). Polymeric nanoparticles for increasing oral bioavailability of Curcumin. Antioxidants, 7(4), 46. https:// doi.org/10.3390/antiox7040046
Witkin, J., & Li, X. (2013). Curcumin, an Active Constiuent of the Ancient Medicinal Herb Curcuma longa L.: Some Uses and the Establishment and Biological Basis of Medical Efficacy. CNS & Neurological Disorders - Drug Targets, 12(4), 487–497. https://doi.org/10.2174/1871527311312040007
Xie, X., Tao, Q., Zou, Y., Zhang, F., Guo, M., Wang, Y., … Yu, S. (2011). PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. Journal of Agricultural and Food Chemistry, 59(17), 9280–9289. https://doi.org/10.1021/jf202135j










