
16 minute read
Chronic Jet Lag and Its Long Term Effects on Brain Function
Michelle Wrona
Frequent transmeridian travel has the potential to have a detrimental effect on circadian rhythms and brain function. This idea is commonly known as jet lag, which often has a greater influence on travelers undergoing a phase advance, which relates to eastbound travel, instead of phase delays, instigated by westbound travel. Researchers are seeking to determine the effects of chronic jet lag simulation on adult male Sprague Dawley rats. Specifically, they speculate that chronic jet lag negatively influences cognitive and affective behaviours, and hippocampal neurogenesis. The three randomly assigned experimental groups utilized included a control group, phase advancement group, where the light period was shortened by 6 hours, and a phase delay group, whose dark period increased by 6 hours. The light-dark (LD) cycle shifts occurred over a span of eight weeks, with each shift occurring once a week. After the eighth shift, the rats underwent a variety of tests, including: sucrose consumption testing, open field testing, elevated plus maze, forced swim test (FST), and object recognition testing. These tests were conducted to evaluate alterations in emotional behaviour, specifically in anxiety and depression-like symptoms, and to examine short-term memory and learning. It was implied that phase advances had a negative effect on the rats, signifying that it enhances depressive behaviours and deficits in memory. Further discussions showcased that phase advance rats had reduced DCX+ cells in the hippocampus, in addition to disrupted object retention memory. Additionally, symptoms of depression were seen after phase advances. Furthermore, phase delays had little to no effect on the measures examined.
Advertisement
Key words: chronic jet lag; light dark (LD) cycle; circadian rhythms; phase advance; phase delay; hippocampal neurogenesis; depression; anxiety
Background and Introduction
Jet lag is a condition experienced by humans shifting time zones due to travel, leading to the lack of synchrony between the time in the new time zone and the body’s clock (Shen et al., 2019). Several factors, including the direction travelled, timing of travel, and number of time zones shifted, have an impact on the severity of this condition (Choy and Salbu, 2011). Symptoms include lack of concentration, lethargy, disorders impacting the gastrointestinal tract, irritability, and difficulties with sleep (Zhang et al., 2020).
This condition impacts the body’s circadian “clock,” which, over time, will likely acclimatize to the new time zone (Choy and Salbu, 2011). When experienced frequently, this condition may become chronic, leading to cognitive deficits and depression. Depressive behaviours are likely instigated due to the hypothalamic-pituitary-adrenal (HPA) axis causing the exogenous administration of CORT, a corticosteroid which regulates stress (Shen et al., 2019). It is also suggested that the central circadian clock impacts cognitive functions such as learning and memory to the extent that the greater the phase advance, the greater impact on memory (Loh et al., 2010). implies that humans travelling westbound are less likely to experience damaging effects on brain function.
Major Results
Memory Impairments in Jet Lag-Influenced Rats
To investigate the effects of experimental chronic jet lag on memory, Horsey and her colleagues performed a novel object recognition test. It was discovered that after a 60-minute retention interval, phase advance rats had a lower discrimination index than control and phase delay rats. They demonstrated that chronic jet lag in rodents induces impairments in object recognition memory, suggesting that the impairments specifically occur in the stabilization of new memories. This stabilization is normally controlled by long-term potentiation and synaptic efficacy, though, due to circadian disruption, instability resulted. This memory impairment is induced by stress level cortisol (Cho et al., 2000), meaning that exposure to this hormone has the potential to result in memory deficits. Phase delays had little to no effect on the rodent’s memory and learning skills.
Although multiple studies demonstrate the negative effects of jet lag, clinical studies have rarely focused on the decline in neurogenesis in animal models, especially in those sharing similarities in brain structures to humans. Horsey et al.’s study highlighted the decrease in hippocampal neurogenesis through immunohistochemistry, where immature neurons were viewed using DCX, a doublecortin. The quantity of immature DCX + dendate granule cells was assessed by an optical fractionator method, and later analyzed. This is a significant part of the study which highlights that jet lag has a long-lasting effect on an individual, instead of causing short-term symptoms such as fatigue. This suggests that the Horsey et al. study has the potential to be revolutionary, allowing individuals to view the major findings initially seen in rats as something that may affect everyone.
While it is known that jet lag can have chronic effects on brain function, specific tests on animal models have not been conducted to physically view these behaviours. Horsey et al. were aware of the negatively associated impacts of chronic jet lag, though their purpose was to seek physical evidence of deficits in brain function and the decrease of neurogenesis. By viewing decreased neurogenesis in the hippocampus, disrupted object retention memory, body weight changes in phase advance rats, the authors could conclude that jet lag, especially in animals experiencing phase advancements by at least 6 hours, has a detrimental effect. They determined that the consequences of phase advances include an increase in anxiety and depression, memory impairments, and neurogenesis disruption. These findings provide a link between phase advances and increased harmful effects on brain function and overall cognition. This Other studies also suggest that memory impairments have a relationship with the decline in hippocampal neurogenesis (Gibson et al., 2010). After chronic temporal disruption procedures, rodents were unable to learn a specific task and demonstrate preference amongst a rewarding versus unrewarding chamber.
Adapted from Gibson, Erin M., Connie Wang, Stephanie Tjho, Neera Khattar, and Lance J. ’ ‘ Jet Lag “ Experimental Kriegsfeld. 2010. Inhibits Adult Neurogenesis and Produces Term Cognitive Deficits in Female Ham- - Long
Edited by Shin Yamazaki. PLoS ONE 5 ” sters.
https://doi,org/10.1371/ (12): e15267.
Figure 1 A) control versus jet lagged (JL) hamsters; control show preference for black chamber, JL show no preference (B) after training; control rodents show preference for rewarding chamber, JL show no preference; JL return to standard LD cycle (C) after more training; control rodents show same preference; JL show no preference; training began in chambers opposite to first test (D) JL did not learn new task; control rodents preferred new chamber
Increase in Anxiety and Depressive Behaviours in Jet Lag
Influenced Rats
It is hypothesized that melatonin has a role in alleviating jet lagrelated symptoms. In individuals with depression, there are aberrations in the secretion of this hormone, leading to low 191
levels (Katz et al., 2001). In many studies, researchers focus on the phase shift hypothesis (PSH), which indicates that a phase shift in the LD cycle can induce depression in an individual (Lewy et al., 2007). In Horsey et al.’s study, evidence from several behavioural measures studying phase shifts in LD cycles support the relationship between the increase in anxiety and depression and disruptions in circadian rhythms. Horsey and her colleagues utilized a sucrose consumption test in their study to test for anhedonia in the male rodents. In the study, decreased sucrose consumption was demonstrated in the rodents subjected to weekly phase advances in comparison to phase delays.
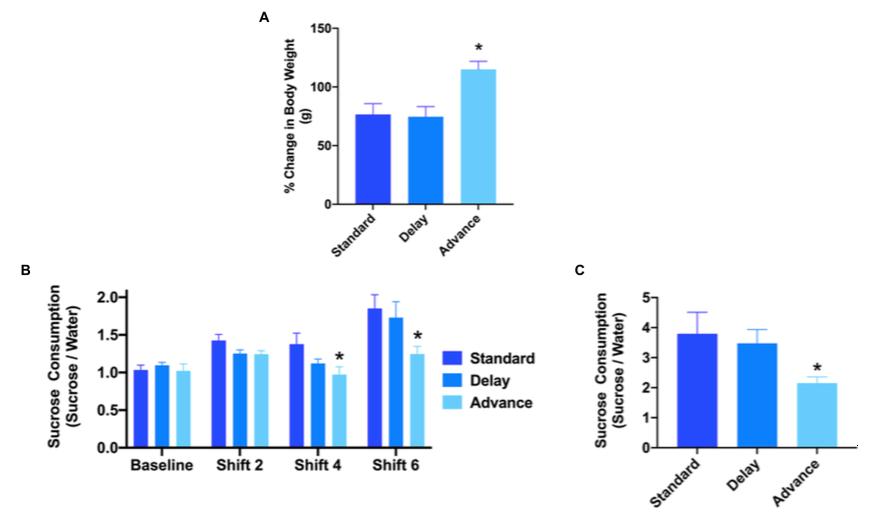
Studies have shown that high stress due to increased cortisol levels and jet lag have a role in the induction of hippocampal atrophy, leading to deficits in learning and memory (Cho, 2000). It is predicted that circadian disruption has the detrimental effect of reducing neurogenesis and hippocampal cell proliferation, which accounts for the cognitive deficits previously explained. Deficits from the reduction in neurogenesis have been hypothesized to occur after the termination of jet lag, meaning that this condition causes long-term negative effects on the brain (Gibson et al., 2010). The Horsey et al. study also demonstrated the same idea, implying that phase advances have the potential to act as “stressors” that decrease hippocampal neurogenesis. In the study, researchers sought to find the total number of DCX + cells in rodents, and compare the number amongst the experimental groups. In phase advance rats, this number was significantly lower than phase delay rodents or those on a standard LD cycle.
Adapted from Horsey, Emily A., Teresa Maletta, Holly Turner, Chantel Cole, Hugo Lehrman, and Neil M. Fournier. 2020. “Chronic Jet Lag Simulation Decreases Hippocampal Neurogenesis and Enhances Depressive Behaviors and Cognitive Deficits in Adult Male Rats.” Frontiers in Behavioral Neuroscience 13 (January). https://doi.org/10.3389/fnbeh.2019.00272.
Figure 2 A) Change in body weight in rodents of three experimental groups; phase advanced rats weight increased significantly more than standard and phase delays rodents. (B) Phase advanced rats consumed less sucrose after shifts 4 and 6 than standard and phase delay rats. (C) Sucrose consumption over a 24 hr period after shift 8 ended in (B). Phase advanced rats consumed less sucrose than standard and phase delayed rats.

Adapted from Horsey, Emily A., Teresa Maletta, Holly Turner, Chantel Cole, Hugo Lehrman, and Neil M. Fournier. 2020. “Chronic Jet Lag Simulation Decreases Hippocampal Neurogenesis and Enhances Depressive Behaviors and Cognitive Deficits in Adult Male Rats.” Frontiers in Behavioral Neuroscience 13 (January). https:// doi.org/10.3389/fnbeh.2019.00272.
Similar behavioural measures were studied in the FST, where phase advance rats experienced greater immobility than the other two groups. Similar findings were seen in the open field arena and elevated plus maze, alluding to decreased exploration of areas being related to increased anxiety levels. The study implies that phase advances influence mood regulation and anxiety (Horsey et al., 2020).

Figure 4 Estimation of the quantity of DCX + cells in the dendate subgranular zone. Chronic phase advance rats had a significantly reduced amount of DCX + cells in comparison to standard LD cycle rodents or phase delay rodents.
The authors highlight that DCX is not represented solely during early neuronal differentiation, but throughout the entire maturation (Horsey et al., 2020). Chronic jet lag affects immature (DCX +) neuronal maturation due to phase advances causing a loss of late stage DCX + cells. This indicates that the process of phase advance likely obstructs cell maturation and survival. The loss of these late stage cells in the hippocampus associates with weakened learning and memory. Moreover, Horsey and her colleagues imply that increased stress and corticosteroid levels decrease the number of late stage DCX + cells, reducing dendritic complexity of immature granule cells.
Adapted from Horsey, Emily A., Teresa Maletta, Holly Turner, Chantel Cole, Hugo Lehrman, and Neil M. Fournier. 2020. “Chronic Jet Lag Simulation Decreases Hippocampal Neurogenesis and Enhances Depressive Behaviors and Cognitive Deficits in Adult Male Rats.” Frontiers in Behavioral Neuroscience 13 (January). https://
Figure 3 A) FST; time immobile on day 1 of a 15 min test. (B) Time immobile on day 2 of a 5 min test. Phase advanced rodents spent more time immobile than other experimental groups on day 2.
Conclusion/Discussion
Horsey et al. (2020) concluded that their findings demonstrate a link between chronic jet lag and detrimental deficits in cognition, memory, and ones causing anxiety and depressive behaviours. By conducting several tests on adult male rats after inducing chronic jet lag for eight weeks, the authors demonstrated
that phase advances lead to a decline in hippocampal neurogenesis, which, in turn, has a negative effect on object retention memory and learning, and increases anxiety and depression. Phase delays have limited effects on behaviour and neurogenesis, emphasizing that chronic jet lag likely contributes to negative long-lasting effects in eastward travel. evidence. There is a suggestion that phase advances can have differing effects on females due to a contrast in emotional responses to stimuli. While other studies have focused on jet lag simulations on female rodents (Gibson et al., 2010), the differences between male and female behaviours were not studied, thus making this an important experiment to be conducted.
Furthermore, the authors acknowledge that there are other explanations for the increase in cognitive deficits in the rodent brain due to chronic circadian disruption. One is the idea that circadian clock gene expression is likely disturbed due to phase advances of the LD cycle (Horsey et al., 2020). Phase advances are also hypothesized to impact glucocorticoid secretion and the response to stress more than phase delays, signifying that phase advances can induce chronic stress. The authors state that chronic stress has an unfavourable impact on molecular mechanisms, especially those involved in the hippocampus, amygdala and nucleus accumbens, brain regions that impact behaviour, memory and learning. If the hippocampus is directly affected, this implies that stress can instigate the absence of maturation of adult-born neurons, which was seen in this study.
The theory that hippocampal neurogenesis is specifically impacted by chronic jet lag is one that has not been deeply studied by other researchers. Horsey and her colleagues explore various negative effects of chronic jet lag, though a decline in hippocampal neurogenesis seems to be the spark that affects all the other behaviours seen in the male rodents, including cognitive deficits in memory. While there is a plethora of evidence suggesting that the hippocampus has an essential role in spatial memory and being a synaptic basis for learning (Iggena et al., 2017; Bliss and Collingridge, 1993), the authors in this study suggest that more evidence of the role of adult hippocampal neurogenesis is required to fully comprehend the implications of decreased neurogenesis due to jet lag. Horsey and her colleagues identified impairments in the hippocampus on a surface level view, though disrupted neurogenesis has a greater impact on brain function than what is currently known.
Future Directions
Researchers should look at performing an identical experiment as to Horsey et al.’s, but instead, utilize female rats. This will allow for greater focus on potential sex differences that possibly involve emotional responses. By creating an identical study, researchers will eliminate any error and can see if females experience the same effects as male rodents. It is predicted that females will likely experience a similar effect in memory and neurogenesis, but a differing impact of the development of mood disorders. This is an essential study that must be conducted to see if all animal models experience the same effects of jet lag, and if this can be further applied to humans.
Critical Analysis
While Horsey et al. (2020) provide revolutionary results on the negative effects of chronic jet lag on rodents, there is still much to be explored in this field and more experiments to be conducted to make accurate conclusions. After reviewing this paper, one concept to be explored is the influence of chronic jet lag on mood disorder development. While the authors highlighted that chronic phase advances leads to an increase in anxiety and depressive behaviours, there is an absence of information pertaining to the development of these behaviours and what axes or pathways jet lag impacts to instigate the disorders. Studies (Katz et al., 2001) publish the psychiatric aspects of jet lag by reviewing the symptoms of mood disorders, though very few have been able to determine the molecular mechanisms circadian disruptions impair to cause these disorders.
Another possible study to be conducted is one to decide if sex differences exist in behavioural responses to jet lag. In this study, Horsey et al. (2020) explore jet lag simulation effects on adult male rats. However, the authors acknowledge that their data is limited in making conclusions due to the lack of female Seeking the effects of jet lag on human hippocampal neurogenesis would be the most important part of the study to mimic. Viewing the impact would allow researchers to learn more about the hippocampus as a brain region in general. By using procedures such as immunohistochemistry, hippocampal brain slices can be viewed. While different tests should be carried out, such as self-report tests indicating anxiety and depression instead of a FST, which would not be effective in humans, the same impairments can be tested for.
This study focused on the increase in depressive and anxiety behaviours in male rodents phenotypically. By genotyping rodents and viewing the alterations in circadian clock gene expression over time, the authors can make concrete conclusions about their results. A way to view the change in gene expression over time is to track mRNA oscillations of clock genes (Waddington Lamont et al., 2007), which present 24-hour rhythms in the SCN. After or during the jet lag simulation of animal models, oscillations can be tracked over time and compared to pre-jet lag levels. It is expected that oscillations will change especially due to phase advances, affecting gene expression.
1.
2.
3. 4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15. Cho, Kwangwook, A. Ennaceur, Jon C. Cole, and Chang Kook Suh. 2000. “Chronic Jet Lag Produces Cognitive Deficits.” Journal of Neuroscience 20 (6): RC66–RC66. https://doi.org/10.1523/JNEUROSCI.20-06-j0005.2000. Cho, Kwangwook. 2001. “Chronic ‘Jet Lag’ Produces Temporal Lobe Atrophy and Spatial Cognitive Deficits.” Nature Neuroscience 4 (6): 567–68. https://doi.org/10.1038/88384. Choy, Mary, and Rebecca L. Salbu. 2011. “Jet Lag.” Pharmacy and Therapeutics 36 (4): 221–31. Davidson, Alec J., Oscar Castanon-Cervantes, Tanya L. Leise, Penny C. Molyneux, and Mary E. Harrington. 2009. “Visualizing Jet Lag in the Mouse Suprachiasmatic Nucleus and Peripheral Circadian Timing System.” European Journal of Neuroscience 29 (1): 171–80. https://doi.org/10.1111/j.1460-9568.2008.06534.x. Deacon, S., and J. Arendt. 1996. “Adapting to Phase Shifts, I. An Experimental Model for Jet Lag and Shift Work.” Physiology & Behavior 59 (4–5): 665–73. https://doi.org/10.1016/0031-9384(95)02147-7. Gibson, Erin M., Connie Wang, Stephanie Tjho, Neera Khattar, and Lance J. Kriegsfeld. 2010. “Experimental ‘Jet Lag’ Inhibits Adult Neurogenesis and Produces Long-Term Cognitive Deficits in Female Hamsters.” Edited by Shin Yamazaki. PLoS ONE 5 (12): e15267. https://doi.org/10.1371/journal.pone.0015267. Horsey, Emily A., Teresa Maletta, Holly Turner, Chantel Cole, Hugo Lehmann, and Neil M. Fournier. 2020. “Chronic Jet Lag Simulation Decreases Hippocampal Neurogenesis and Enhances Depressive Behaviors and Cognitive Deficits in Adult Male Rats.” Frontiers in Behavioral Neuroscience 13 (January). https:// doi.org/10.3389/fnbeh.2019.00272. Iggena, Deetje, York Winter, and Barbara Steiner. 2017. “Melatonin Restores Hippocampal Neural Precursor Cell Proliferation and Prevents Cognitive Deficits Induced by Jet Lag Simulation in Adult Mice.” Journal of Pineal Research 62 (4): 1-12. https://doi.org/10.1111/jpi.12397.
Katz, G. R., Durst, R., Zislin, Y., Barel, Y., and Knobler, H.Y. 2001. “Psychiatric Aspects of Jet Lag: Review and Hypothesis | Elsevier Enhanced Reader.” Medical Hypotheses 56 (1): 20-23. https://doi.org/10.1054/ mehy.2000.1094. Kolla, Bhanu P., and R. Robert Auger. 2011. “Jet Lag and Shift Work Sleep Disorders: How to Help Reset the Internal Clock.” Cleveland Clinic Journal of Medicine 78 (10): 675–84. https://doi.org/10.3949/ccjm.78a.10083. Lamont, Elaine Waddington, Francine O. James, Diane B. Boivin, and Nicolas Cermakian. 2007. “From Circadian Clock Gene Expression to Pathologies.” Sleep Medicine, Circadian Rhythms in Sleep Medicine, 8 (6): 547–56. https://doi.org/10.1016/j.sleep.2006.11.002. Lewy, Alfred J., Jennifer N. Rough, Jeannine B. Songer, Neelam Mishra, Krista Yuhas, and Jonathan S. Emens. 2007. “The Phase Shift Hypothesis for the Circadian Component of Winter Depression.” Dialogues in Clinical Neuroscience 9 (3): 291–300.
Loh, Dawn H., Juliana Navarro, Arkady Hagopian, Louisa M. Wang, Tom Deboer, and Christopher S. Colwell. 2010. “Rapid Changes in the Light/Dark Cycle Disrupt Memory of Conditioned Fear in Mice.” PLoS ONE 5 (9). https://doi.org/10.1371/journal.pone.0012546. Shen, Qichen, Junli Wu, Yuehan Ni, Xiaoxian Xie, Chunan Yu, Qingfeng Xiao, Jiafeng Zhou, Xia Wang, and Zhengwei Fu. 2019. “Exposure to Jet Lag Aggravates Depression-like Behaviors and Age-Related Phenotypes in Rats Subject to Chronic Corticosterone.” Acta Biochimica et Biophysica Sinica 51 (8): 834–44. https:// doi.org/10.1093/abbs/gmz070. Zhang, Feifei, Weikai Li, Huiru Li, Shaobing Gao, John A. Sweeney, Zhiyun Jia, and Qiyong Gong. 2020. “The Effect of Jet Lag on the Human Brain: A Neuroimaging Study.” Human Brain Mapping 41 (9): 2281–91. https:// doi.org/10.1002/hbm.24945.










