
16 minute read
Human Amniotic Epithelial Stem Cells Aa A Therapy For Alzheimer’s Disease
Reetu Khan
Alzheimer’s disease (AD) is an irreversible neurodegenerative condition associated with cognitive decline in older populations 1 . Pathological hallmarks of this disorder include the accumulation of amyloid plaques, cerebral angiopathy and the loss of neuronal and synaptic function 2 . Despite being the most common cause of dementia, there is currently no cure for AD 2 . Modern therapies aim to cease progression of the disease. One novel treatment that has recently been brought to light is stem cellbased therapy. Kim et al. (2020) study the therapeutic benefits of human amniotic epithelial cells (hAESCs) on a transgenic mouse model of AD 3 . Upon receiving intracerebral injections of either hAESC or vehicle, Tg2576 transgenic mice and wildtype mice performed a set of behavioral tests to assess spatial and working memory. In the Morris water maze test, Tg2576-hAESC mice had shorter escape latencies than Tg2576-vehicle mice. In the Y-Maze test, Tg2576-hAESC mice displayed higher rates of spontaneous alternation compared to Tg-vehicle treated mice. Injection of hAESCs reduce Beta-secretase (BACE) activity, the protein responsible for development of amyloid plaque generation 4 , and improve cognitive function. These findings illustrate the potential hAESCs carry as a future therapeutic agent.
Advertisement
Key words: Alzheimer’s disease (AD), human amniotic stem cells (hAESCs), BACE, amyloid plaques, neurofibrillary tangles, transgenic Tg2576 mouse model .
Background and Introduction Alzheimer’s disease (AD), a progressive neurodegenerative disorder, is the prevalent cause of dementia following senescence 3,5 . It is characterized by memory loss, language impairment, attention-deficits and cognitive decline 1 . Histological markers of AD include the combined presence of amyloid-b (Ab) plaques and neurofibrillary tangles containing hyperphosphorylated and misfolded tau protein 2 . Amyloid precursor protein (APP) is sequentially cleaved by b- and g-secretases, resulting in the accumulation of beta amyloid aggregates. These manifestations lead to microtubule collapse, thus impairing structural support and axonal transport and leading to neuronal death 6 . The buildup of amyloid plaque occurs before the onset hAESC or vehicle in the dentate gyri of the bilateral hippocam
of cognitive dysfunction 2 , thus making early diagnosis a challenge. Despite its prevalence in individuals over the age of 65 1 , a cure for AD is yet to be found. Current pharmacological treatments, such as the cholinesterases donepezil and rivastigmine, are prescribed for mildmoderate AD to slow its progression 7 . While the mechanisms are still unknown, researchers believe these medications prevent the breakdown of acetylcholine, a molecule important for memory and learning 7 . N-methyl-D-aspartate (NMDA) antagonists are another form of treatment that regulate glutamatergic activity and block excessive levels from eliciting cell death 7 . These drugs, however, have limited efficacy and simply allevispatial learning and memory 3,16 . Rodents navigate around the
ate symptoms without providing long-lasting relief 8 . Stem cell-based therapy has been a focus of interest in the ongoing search for neurodegenerative cures. Several studies have outlined their therapeutic effects on spinal cord injury repair 9 , amyotrophic lateral sclerosis 10 and Huntington’s disease 11 . Novel methods aim to target degenerating neuronal networks observed in AD to induce regeneration and repopulation 3 . In order to develop a practical stem-cell based therapy, cells must be extracted from the most appropriate source. These may include pluripotent embryonic stem cells involved in the development of germ layers, mesenchymal stem cells capable of forming mesenchymal tissue, neural stem cells responsible for growth of neural cell types or human amniotic epithelial stem cells (hAESCs) 6 . These stem cells are derived from the innermost layer of the placenta. They bear the ability to differentiate like embryonic stem cells while also retaining immunomodulatory properties of adult stem cells 12 . Furthermore, studies claim they are able to synthesize neurotrophic and growth factors essential for neuronal regeneration and survival 13 . In a study conducted by Xue et al., transplantation of hAESCs into double transgenic mice expressing APP increased hippocampal acetylcholine levels and enhanced spatial memory 14 . This effect is quite similar to those exerted by pharmacological cholinesterase treatments, suggesting that hAESCs may function through a similar mechanism and serve as a promising candidate for the treatment of AD. In the original paper, Kim et al. explored the potential therapeutic benefits of hAESCs in a mouse model of AD. In their study, four groups of mice were experimented on: Tg2576 transgenic mice treated with either hAESC (Tg-hAESC) or vehihAESC (WT-hAESC) or vehicle (WT-vehicle). Tg2576 transgenic mice were used model AD. These mice overexpress mutant amyloid precursor protein (APP) to mimic elevated levels of Ab and plaque central to AD 15 . The mice performed two sets of behavioral tests to assess spatial and working memory. TghAESC mice exhibited decreased escape latencies and higher rates of spontaneous alternation, signifying better spatial and working memory than Tg-vehicle mice. These findings highlight the important neurological mechanisms by which hAESC transplantation may influence amyloid burden and BACE activity to improve cognitive decline in AD. Understanding and exploring these mechanisms may give rise to a novel treatment or cure.
Major Results At 11 months of age, mice received intracerebral injections of pus. Stereotactic injections were administered relative to the bregma at the coordinates: AP = −0.15 mm, ML = ±0.13 mm, DV = −0.19 mm. At 14 months, all four groups of mice performed two sets of behavioral tests to assess cognitive function: the Morris water maze test and the Y-maze test.
--- hAESCs Improve Spatial Learning: Morris Water Maze ---
The Morris water maze is the gold standard test used to assess cle (Tg-vehicle) and age-matched wildtype mice treated with
perimeter of an open pool, using distal cues and memory, in order to locate a hidden escape platform beneath the water 16,17 . The time required to correctly locate the platform is measured as the rodent’s escape latency 17 . Overall, Tg mice displayed significantly longer escape latencies compared to the WT mice. Tg-hAESC mice, however, showed decreased escape latencies than Tg-vehicle mice, indicating improved spatial memory. Both WT groups showed no significant differences in escape latency time (Figure 1A).
To determine if the groups had memorized the location of the platform in zone 4, a probe trial was performed 48 hours after the final trial. In this trial, the mice were able to swim freely without the platform 17 . Tg-hAESC mice showed significantly recovered escape latencies than the Tg-vehicle mice; in fact, their latency times closely matched the latency times of the WT groups (Figure 1B). Both WT groups and the Tg-hAESC group spent more time in zone 4 than zones 1-3.
Figure 1. A) The Morris water maze test was performed for six consecutive days, three months after mice received intracerebral injections. Escape latency times were recorded. B) 48 hours after their final trial, a probe test was conducted. Escape latency times were recorded. Figure origin: Kim, Ka Young, Yoo-Hun Suh, and Keun-A Chang. Therapeutic Effects of Human Amniotic Epithelial Stem Cells in a Transgenic Mouse Model of Alzheimer’s Disease. April 10, 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7178120/pdf/ ijms-21-02658.
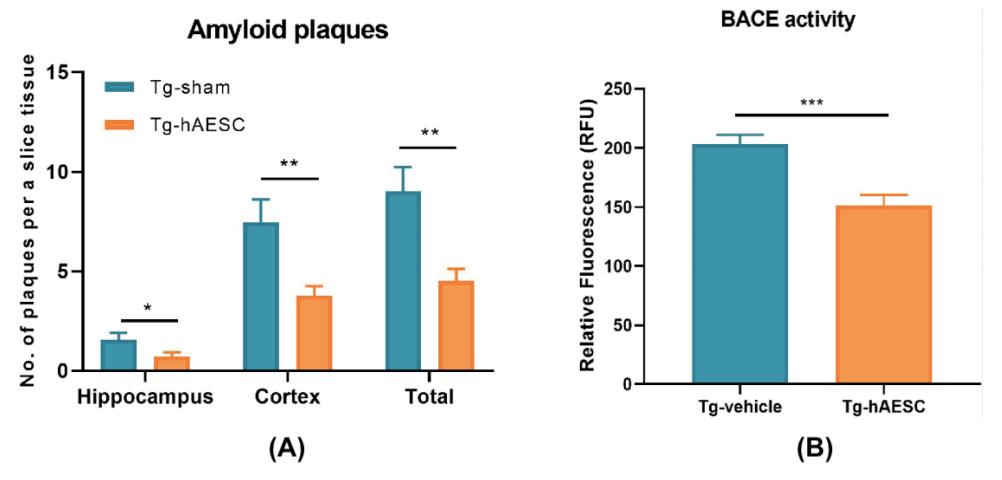
--- hAESCs Improve Working Memory: Y-Maze Test --- Figure 3. A) The number of plaques per a slice tissue were rec
The Y-Maze is a learning and memory test that assesses the tendency of a rodent to explore a new environment 18 . The test measures how often the rodent enters a different arm of the maze instead of returning to the one it previously visited; this is known as spontaneous alternation 11 . The total number of arm entries remained the same between groups (Figure 2A), however, Tg-hAESC mice exhibited significantly higher rates of spontaneous alternation than Tg-vehicle mice (Figure 2B). Conclusions/Discussions As researchers advance in their knowledge on the etiology of AD, a well-established long-term cure remains to be discovered. As of now, current therapies include cholinesterases and NMDA antagonists, which only halt the progression of the disease 7 . However, stem cell-based therapy appears to be revolutionizing the field. hAESCs, stem cells derived from the placenta, can differentiate into cells of the germ layer, such as the endoderm, mesoderm and ectoderm 3 . Previous studies have examined the role of these cells and discovered that intravenous injection of hAESCs into APPswe mice correlated with fewer amyloid plaques and improved spatial learning 19 . Furthermore, hAESCs have reduced amyloid deposition in C57BL/6J

Figure 2. A) The Y-maze test was performed, three months after mice received intracerebral injections. The total number of arm entries was recorded. B) The rate of spontaneous alternation after performing the Y-maze test was recorded. Figure origin: Kim, Ka Young, Yoo-Hun Suh, and Keun-A Chang. Therapeutic Effects of Human Amniotic Epithelial Stem Cells in a When compared to Tg mice treated with vehicle, Tg-hAESC mice exhibited exceptionally better spatial memory and workhAESCs successfully alleviated cognitive decline in transgenic
Transgenic Mouse Model of Alzheimer’s Disease. April 10, 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7178120/pdf/ ijms-21-02658.
--- hAESCs Reduce BACE Activity ---
To examine the abundance of amyloid present in Tg and WT mice, their cortices and hippocampi were stained with Congo red. Tg-hAESC mice displayed significant reductions of amyloid plaques compared to the Tg-vehicle group overall (Figure 3A). Beta-secretase (BACE) activity was analyzed as well to gain an understanding of the amyloid plaque reduction observed upon hAESC transplantation. BACE activity increased over time in all orded in Tg-hAESC and Tg-vehicle mice. B) BACE activity was recorded in Tg-vehicle and Tg-hAESC mice. Figure origin: Kim, Ka Young, Yoo-Hun Suh, and Keun-A Chang. Therapeutic Effects of Human Amniotic Epithelial Stem Cells in a Transgenic Mouse Model of Alzheimer’s Disease. April 10, 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7178120/pdf/ ijms-21-02658.
APP by attenuating oxidative stress and stimulating antioxidative enzymes 20 . These observations are in line with the results obtained in the current study, further elucidating the therapeutic effects of hAESCs.
In this study, transplantation of hAESCs into the bilateral hippocampus of Tg2576 transgenic mice reduced BACE activity and amyloid plaque burden, thus improving cognitive impairment. ing memory. The authors concluded that transplantation of four Tg and WT groups, however, decreased activity was de
mouse models of AD, suggesting hAESCs could be a novel therapeutic treatment for AD memory impairment.
Additionally, the authors interpret that reduced BACE activity could be the mechanism by which hAESCs exert their effects to reduce amyloid plaque burden in Tg2576 mice 3 . BACE is an en-
enzyme responsible for the proteolysis of APP, leading to production of the N-terminus of Ab 4 . It has been the prime drug target for AD. Studies using BACE1 knockout (BACE1 -/- ) mice demonstrated that when these mice were bred with APP transgenic mice, Ab generation, amyloid burden and cognitive deficits were prevented 21 . Moreover, inhibition of BACE has been shown to stop the generation of Ab 3 , thus making it a potential therapeutic approach to treating AD. Not only do these important revelations shed light the unknown processes of hAESC transplantation and their effects on BACE activity, they lead scientists closer to finding a potential cure for AD. Octodon Degus mice and wildtype mice will either be injected used in place of transgenic Tg2576 mice. Octodon degus mice naturally display AD-like pathologies and share a close bA sequence homology with humans 27 , making them an ideal candidate to physiologically model sporadic AD. Compared to Tg2576 mice that only display intraneuronal Ab and parenchymal Ab plaques, Octodon Degus mice exhibit a full range of neuropathological features: intraneuronal Ab, parenchymal Ab plaques, hyperphosphorylated tau, neurofibrillary tangles, and synaptic dysfunction 27 . It is unclear if neuronal loss is present in these animals 27 .
Critical Analysis Kim et al. effectively examine the potential short-term and long -term benefits of stem-cell transplantation, however, they fail to acknowledge the possibility of graft/transplantation rejection. Non-autologous hAESCs incur the risk of immune rejection 22 , thus rendering them ineffective for some individuals. While hAESCs are less susceptible to rejection than other types of stem cells, due to their low immunogenicity 23 , a study conducted by Chiavegato et al. observed the rejection of amniotic stem cells in an immunodeficient/immunosuppressed rat modand a consequence of this mechanism is the alleviation of cogof hAESCs, it is plausible and might indicate that hAESCs have no therapeutic influence on AD.
el 24 . They concluded that the xenotransplantation of amniotic stem cells in cell therapy is hindered by their immunogenic factors and phenotypic unpredictability 24 . One possible solution may be to use immunosuppressive drugs, however, these may induce unwanted side effects 25 or introduce variability to the study.
The potential of hAESCs in the treatment of AD becomes evident in transgenic mouse models, however, it is questionable whether these model organisms are representative of the human body. While most cases of AD occur sporadically in a population of genetically heterogenous individuals 6,26 , transgenic mouse models are based on familial mutations in genetically homogenous populations 14 . Transgenic 2576 mice used in this study do not exhibit the neuronal loss characteristic of human AD 6,26 and therefore do not fully replicate the human disease. Future AD studies may need to experiment with higher-order animals that accurately embody the clinical features of the disease.
Furthermore, the authors primarily studied the prefrontal coraffected by the development of amyloid plaques and neurofibrillary tangles. However, neurofibrillary tangles can further accumulate in the amygdala and thalamus, eventually spreading to associative isocortical regions 2 . These are possible brain structures to study in upcoming experiments and reviews to fully understand the neurological etiology of AD. with hAESCs or vehicle in the bilateral hippocampus. Three months later, their cognitive function will be assessed through a set of behavioral tests. Upon completing the tests, different stains will be used to analyze their hippocampi, prefrontal and entorhinal cortices. One stain will identify amyloid plaques, another stain will identify neurofibrillary tangles, and so forth –they will examine whether levels have been upregulated, downregulated or remained the same (baseline measurements will be taken prior to injection of hAESCs or vehicle).
If amyloid burden, neurofibrillary tangles and hyperphosphorylated tau levels decrease in Octodon Degus-hAESC mice, we should expect to see them exhibit improved spatial and working memory compared to Octodon Degus-vehicle mice. This may suggest that there is some underlying mechanism by which hAESCs are reducing amyloid and neurofibrillary tangle burden, nitive decline. One possible mechanism could be that hAESCs are secreting neural growth factors that are reconstructing pathways in memory-related areas of the brain. Alternatively, they could be secreting factors that inhibit the formation of amyloid plaques and neurofibrillary tangles or inhibit kinases responsible for the hyperphosphorylation of tau 3 . If amyloid burden, neurofibrillary tangles and hyperphosphorylated tau levels increase or remain the same upon transplantation, we would expect to see Octodon Degus-hAESC mice performing just as poorly as Octodon Degus-vehicle mice. Their spatial and working memory will remain impaired. While this is an unlikely outcome, based on the reported beneficial effects tex, entorhinal cortex and hippocampus, the dominant areas
In similar experiments, perhaps other areas of the brain can be investigated, such as the amygdala, thalamus, and associative isocortex. When staining for amyloid plaques, hyperphosphorylated tau and neurofibrillary tangles, we should expect to see levels decrease in these particular areas, too. Overall, these studies should demonstrate how hAESCs can alleviate cognitive decline in AD and direct us toward a human cure.
Future Directions In future experiments, perhaps Octodon Degus mice can be
1. “What Is Alzheimer’s Disease?” n.d. National Institute on Aging. Accessed June 18, 2020. https://www.nia.nih.gov/health/whatalzheimers-disease. 2. Serrano-Pozo, Alberto, Matthew P. Frosch, Eliezer Masliah, and Bradley T. Hyman. 2011. “Neuropathological Alterations in Alzheimer Disease.” Cold Spring Harbor Perspectives in Medicine: 1 (1). https://doi.org/10.1101/cshperspect.a006189. 3. Kim, Ka Young, Yoo-Hun Suh, and Keun-A Chang. 2020. “Therapeutic Effects of Human Amniotic Epithelial Stem Cells in a Transgenic Mouse Model of Alzheimer’s Disease.” International Journal of Molecular Sciences 21 (7). https://doi.org/10.3390/ ijms21072658. 4. Vassar, Robert, Dora M. Kovacs, Riqiang Yan, and Philip C. Wong. 2009. “The β-Secretase Enzyme BACE in Health and Alzheimer’s Disease: Regulation, Cell Biology, Function, and Therapeutic Potential.” The Journal of Neuroscience 29 (41): 12787–94. https://doi.org/10.1523/JNEUROSCI.3657-09.2009. 5. Scheltens, Philip, Kaj Blennow, Monique M. B. Breteler, Bart de Strooper, Giovanni B. Frisoni, Stephen Salloway, and Wiesje Maria Van der Flier. 2016. “Alzheimer’s Disease.” Lancet (London, England) 388 (10043): 505–17. https://doi.org/10.1016/S0140- 6736(15)01124-1. 6. Duncan, Thomas, and Michael Valenzuela. 2017. “Alzheimer’s Disease, Dementia, and Stem Cell Therapy.” Stem Cell Research & Therapy 8 (1): 111. https://doi.org/10.1186/s13287-017-0567-5. 7. “How Is Alzheimer’s Disease Treated?” n.d. National Institute on Aging. Accessed June 18, 2020. https://www.nia.nih.gov/ health/how-alzheimers-disease-treated 8. Liu, Alan King Lun. 2013. “Stem Cell Therapy for Alzheimer’s Disease: Hype or Hope?” Bioscience Horizons: The International Journal of Student Research 6 (January). https://doi.org/10.1093/biohorizons/hzt011. 9. Sankar, V, and R Muthusamy. 2003. “Role of Human Amniotic Epithelial Cell Transplantation in Spinal Cord Injury Repair Research.” Neuroscience 118 (1): 11–17. https://doi.org/10.1016/S0306-4522(02)00929-6. 10. Lunn, J. Simon, Stacey A. Sakowski, and Eva L. Feldman. 2014. “Stem Cell Therapies for Amyotrophic Lateral Sclerosis: Recent Advances and Prospects for the Future.” Stem Cells (Dayton, Ohio) 32 (5): 1099–1109. https://doi.org/10.1002/stem.1628. 11. Maucksch, Christof, Elena M. Vazey, Renee J. Gordon, and Bronwen Connor. 2013. “Stem Cell-Based Therapy for Huntington’s Disease.” Journal of Cellular Biochemistry 114 (4): 754–63. https://doi.org/10.1002/jcb.24432. 12. Miki, Toshio. 2018. “Stem Cell Characteristics and the Therapeutic Potential of Amniotic Epithelial Cells.” American Journal of Reproductive Immunology (New York, N.Y.: 1989) 80 (4): e13003. https://doi.org/10.1111/aji.13003. 13. Xu, Huiming, Jiaofei Zhang, Kam Sze Tsang, Hao Yang, and Wei-Qiang Gao. 2019. “Therapeutic Potential of Human Amniotic Epithelial Cells on Injuries and Disorders in the Central Nervous System.” Review Article. Stem Cells International. Hindawi. November 20, 2019. https://doi.org/10.1155/2019/5432301. 14. Xue, Shouru, Chongfang Chen, Wanli Dong, Guozhen Hui, Tianjun Liu, and Lihe Guo. 2012. “Therapeutic Effects of Human Amniotic Epithelial Cell Transplantation on Double-Transgenic Mice Co-Expressing APPswe and PS1ΔE9-Deleted Genes.” Science China. Life Sciences 55 (February): 132–40. https://doi.org/10.1007/s11427-012-4283-1. 15. “Tg2576 | ALZFORUM.” n.d. Accessed June 18, 2020. https://www.alzforum.org/research-models/tg2576. 16. Vorhees, Charles V, and Michael T Williams. 2006. “Morris Water Maze: Procedures for Assessing Spatial and Related Forms of Learning and Memory.” Nature Protocols 1 (2): 848–58. https://doi.org/10.1038/nprot.2006.116. 17. Morris, Richard G. M. 2008. “Morris Water Maze.” Scholarpedia 3 (8): 6315. https://doi.org/10.4249/scholarpedia.6315. 18. “Y Maze Spontaneous Alternation Test | Behavioral and Functional Neuroscience Laboratory | Stanford Medicine.” n.d. Accessed June 18, 2020. https://med.stanford.edu/sbfnl/services/bm/lm/y-maze.html. 19. Kim, Kyung-Sul, Hyun Sook Kim, Ji-Min Park, Han Wool Kim, Mi-Kyung Park, Hyun-Seob Lee, Dae Seog Lim, Tae Hee Lee, Michael Chopp, and Jisook Moon. 2013. “Long-Term Immunomodulatory Effect of Amniotic Stem Cells in an Alzheimer’s Disease Model.” Neurobiology of Aging 34 (10): 2408–20. https://doi.org/10.1016/j.neurobiolaging.2013.03.029. 20. Jiao, Hongliang, Ke Shi, Weijie Zhang, Liang Yang, Lu Yang, Fangxia Guan, and Bo Yang. 2016. “Therapeutic Potential of Human Amniotic Membrane-Derived Mesenchymal Stem Cells in APP Transgenic Mice.” Oncology Letters 12 (3): 1877–83. https:// doi.org/10.3892/ol.2016.4857.
21. Vassar, Robert, and Patty C. Kandalepas. 2011. “The β-Secretase Enzyme BACE1 as a Therapeutic Target for Alzheimer’s Disease.” Alzheimer’s Research & Therapy 3 (3): 20. https://doi.org/10.1186/alzrt82. 22. Hayashi, Yoshihito, Huan-Ting Lin, Cheng-Che Lee, and Kuen-Jer Tsai. 2020. “Effects of Neural Stem Cell Transplantation in Alzheimer’s Disease Models.” Journal of Biomedical Science 27 (1): 29. https://doi.org/10.1186/s12929-020-0622-x. 23. Elias, Maya, Jaclyn Hoover, Hung Nguyen, Stephanny Reyes, Christopher Lawton, and Cesar V Borlongan. 2015. “Stroke Therapy: The Potential of Amniotic Fluid-Derived Stem Cells.” Future Neurology 10 (4): 321–26. https://doi.org/10.2217/FNL.15.19. 24. Chiavegato, Angela, Sveva Bollini, Michela Pozzobon, Andrea Callegari, Lisa Gasparotto, Jenny Taiani, Martina Piccoli, et al. 2007. “Human Amniotic Fluid-Derived Stem Cells Are Rejected after Transplantation in the Myocardium of Normal, Ischemic, Immuno-Suppressed or Immuno-Deficient Rat.” Journal of Molecular and Cellular Cardiology 42 (4): 746–59. https:// doi.org/10.1016/j.yjmcc.2006.12.008. 25. Davulcu, Eren Arslan, and Filiz Vural. 2018. “Immunosuppressive Agents in Hematopoietic Stem Cell Transplantation.” Trends in Transplantation 11 (1). https://doi.org/10.15761/TiT.1000240. 26. Elder, Gregory A., Miguel A. Gama Sosa, and Rita De Gasperi. 2010. “Transgenic Mouse Models of Alzheimer’s Disease.” The Mount Sinai Journal of Medicine, New York 77 (1): 69–81. https://doi.org/10.1002/msj.20159. 27. Castro-Fuentes, Rafael, and Rosario Socas-Pérez. 2013. “Octodon Degus: A Strong Attractor for Alzheimer Research.” Basic and Clinical Neuroscience 4 (1): 91–96.










