
14 minute read
with spinal cord injury
Feature validation and online visualization of forearm high-density EMG in an individual with spinal cord injury
J. Sebastian Correa a , Jordyn E. Ting a , Devapratim Sarma b , Douglas J. Weber a, b a Department of Bioengineering, University of Pittsburgh b Department of Physical Medicine and Rehabilitation, University of Pittsburgh
Sebastian Correa
Sebastian Correa is a bioengineering and Spanish student from Pittsburgh, PA. After graduating, he aspires to combine his passions for neural engineering and improving global health through his future career.
Douglas Weber, Ph.D. is an Associate Professor in the Department of Bioengineering, and he holds secondary appointments in Physical Medicine and Rehabilitation and Electrical Engineering. He is also a faculty member in the Center for the Neural Basis of Cognition, the University of Pittsburgh Brain Institute, and the McGowan Institute for Regenerative Medicine. He established the Rehab Neural Engineering Lab in 2005 when he joined the University of Pittsburgh. Douglas Weber, PhD
Significance Statement
Myoelectric signals can be recorded from the clinically paralyzed muscles of individuals who have been affected by spinal cord injury. With the optimization of signal processing, there is the potential to significantly improve the quality-of-life of patients by allowing them to control assistive devices through the use of these myoelectric signals.
Category: Experimental research Keywords: High-density electromyography, signal feature extraction, spinal cord injury
Abstract
Spinal cord injury (SCI) results in damage to the corticospinal tract, weakening electrically active muscles that generate functional movements. The weak myoelectric signals produced by paretic (weakened) muscles due to SCI can be recorded through high-density electromyography (HDEMG) and used to understand pathological changes related to the injury. A custom HDEMG sleeve was used to measure electromyographic (EMG) signals from the forearms of participants with tetraplegia. Recorded EMG signals were filtered and processed to produce a set of signal features, including the root-mean-square, zero-crossings, and power. These features were used to quantify the strength of activation in forearm muscles which can allow us to discriminate activity patterns associated with different hand movements. The purpose of this study is to optimize the method of processing HDEMG signals with the future goal of enabling people with SCI to intuitively control assistive devices using EMG signals from their clinically paralyzed muscles.
1. Introduction 1.1 Motivation
Every year about 18,000 people in the United States are directly affected by a spinal cord injury (SCI). This amounts to nearly 300,000 people living with spinal cord injuries in the U.S. [1]. This traumatic injury can cause life-long damage to several areas of the body, including motor and sensory impairments. One of the main components in the spinal cord that is responsible for the movement of the limbs is the corticospinal tract. Damage to the corticospinal tract can leave patients with paralysis. Paralysis in all four limbs is known as tetraplegia. As a result, affected individuals are often unable to independently perform activities of daily living and are therefore reliant on caretakers. In this study, we aim to help restore functional movements to individuals affected by SCI through the use of EMG-controlled assistive devices. 1.2 Using Electromyography to Classify Hand Movements Damage to the corticospinal tract weakens electrically active muscles responsible for performing functional movements. Impaired muscle fibers discharge spontaneously, or not at all, due to the pathological deficits found in the spinal cord. This hindered signal is what causes muscles to become paralyzed.
The electrical potentials generated by muscle fibers, known as myoelectric signals, can be measured using electrodes placed at the surface of the skin. This method is known as surface electromyography (EMG) and is commonly used in clinical applications. High-density EMG (HDEMG) electrode arrays have been developed to measure these signals with a higher spatial resolution than traditional EMG devices. These systems use a large number of tightly spaced electrodes to provide a highdensity coverage across the surface of the limb. This allows for a more accurate assessment of deficits in people affected by neuromuscular disorders such as SCI.
Through the processing of these myoelectric signals, there is the potential for the use of this technology to accurately control
assistive devices. While the prospect of giving patients who have been paralyzed a more comfortable life is exciting, there are many obstacles to overcome. EMG signals found in the paretic (weakened) muscles of individuals with SCI are often abnormal and therefore hard to use for the discrimination of activation patterns across hand movements. To overcome this, using our custom HDEMG electrode sleeve array (Fig. 1A), we analyzed EMG signals found in the forearm of a patient with SCI for the accurate classification of hand movements. After processing the data through the use of digital filters, we extracted signal features used to evaluate the strength of the signals and the discriminability between movements. Features used in this analysis were the rootmean-square (RMS), zero-crossings, and power. We then analyzed the features through the use of the signal-to-noise ratio (SNR) and principal component analysis (PCA). This will provide insight into how well our processing methods work for the potential use in neuro-prosthetics. We hope to learn from the results of this study to help improve our method of signal processing, and to eventually help enhance the lives of people affected by SCI.
2. Methods 2.1 Experimental Setup
Our HDEMG electrode sleeve array (Battelle Memorial Institute, Columbus, Ohio) is comprised of 150 monopolar electrodes made from 12 mm diameter steel discs as electrodes with approximately 15 mm inter-electrode spacing (Fig. 1A). Electrodes on the sleeve span from the wrist to the elbow joint (Fig. 1B). This allows for the measurement of signals across the flexor and extensor muscles of the forearm.
The participant with SCI was a 28-year-old male with a C5 motor and C6/C5 (left/right) American Spinal Cord Injury Association Impairment Scale A spinal cord injury. The injury was sustained 9 years prior to the experiment. The able-bodied participant was a 20-year-old male. Only two participants were used for this analysis because only one individual with a SCI was available for testing. All data used in this analysis was recorded in one session for each participant.
The experiment consisted of participants being cued to do several hand movements while wearing the HDEMG electrode sleeve array. Before sessions, the forearm of the participant was cleaned before a conductive gel was applied. During trials, participants were shown one of four different hand movements to do before being indicated to do the movement via a visual cue. The four hand movements used in this analysis include the cylindrical grasp, tripod grasp, lateral grasp, and hand open (Fig. 1C). Movement tasks were chosen because of their common use in daily activities. Movement order was random throughout trials.
Figure 1: HDEMG Sleeve and Movement Task. The HDEMG electrode sleeve uses 150 monopolar channels to measure EMG signals from muscles in the forearm. A. The electrodes in the array are 12 mm diameter steel discs with 15 mm inter-electrode spacing. B. The sleeve spans from the wrist to the elbow joint to measure signals from flexor and extensor muscles across the forearm. C. Hand grasps featured in this paper were chosen because of their common use in daily activities.
2.2 Electromyography Processing
Raw data was collected using an Intan Technologies RHD2000 Recording System (Intan Technologies, Los Angeles, CA). Signals were sampled at a 10 kHz rate across 150 monopolar channels. Monopolar signals at adjacent electrodes were then differenced to remove common noise to create 135 bipolar channels after removing the most proximal row of electrodes. Bipolar signals were then filtered using a 4 th -order digital Butterworth filter. Cutoff frequencies of the band-pass filter were varied to analyze the effects of different frequencies on the discriminability of hand movements. Frequencies tested were between 10-100 Hz and 410-500 Hz for the low and high cutoffs, respectively. This range was chosen in accordance with cutoff frequencies commonly used in analyzing surface EMG data [2]. Data was processed using MATLAB (Mathworks, Natick, MA).
Legend Noise/Rest Region
Signal/Active Region

Figure 2: Data Evolution Data shown comes from one channel of a single trial recorded from both an able-bodied participant and a participant with SCI, respectively. Raw data recorded from the HDEMG sleeve was first differenced to remove noise across common electrodes to create bipolar signals. Bipolar data was then bandpass filtered using a 4 th -order Butterworth filter. Data shown in this figure was filtered using 30 and 450 Hz cutoffs. Features were then extracted using filtered data. The signal-tonoise ratio was calculated using data from the rest and active periods.
2.3 Feature Extraction and Calculation of the Signal-to-Noise Ratio
Signal features can be used to extract useful and dismiss unwanted information found in EMG signals. The selection of features is very important for this to be successful. Features used in this analysis were chosen because of their popularity in EMG signal analysis, their easy implementation, and their range among the time and frequency domains of signal features [3]. Root-Mean-Square (RMS): The root-mean-square (RMS) is a commonly used signal feature found in the time domain. The RMS can be expressed mathematically as [3]:
Zero-Crossings (ZC): The zero-crossings is defined as the number of times the signal passes the zero amplitude axis [4]. It measures frequency information defined in the time domain and it also a commonly used signal feature [3]. The zero-crossings can be expressed mathematically as:
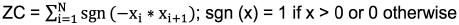
Power: The power was calculated through the use of the power spectral density and is a feature in the frequency domain [3]. The power can be expressed mathematically as: 2.4 Principal Component Analysis
Principal component analysis (PCA) is a statistical procedure used to reduce the dimensionality of a large set of data. It uses eigenvector math to transform the original variables to new variables known as principal components. PCA is commonly used in pattern recognition and EMG signal applications [5]. We used PCA to analyze how well EMG signals could differentiate hand movements. The analysis was performed using a matrix of signal feature data points across all 135 channels. All three features were used in this analysis; however, only RMS data is shown.
3. Results 3.1 Differentiation of Hand Movements
PCA can be used to visualize the differentiation of hand movements resulting from data calculated from signal features. As shown in Fig. 5, there is a clear distinction in the relative spacing amongst each hand movement between the two patients. When analyzing the able-bodied data, movement regions are more defined when compared to the SCI graph. In addition, the variance in the scale of the able-bodied plot is also much larger than in the SCI plot (Fig. 5). These results can also indicate the accuracy of the classification of hand movements for the two groups of data.
Signal features can be visualized in Fig. 3 where they were calculated from one filtered channel of able-bodied and SCI data. Features were calculated using 200 ms overlapping bins with 50% overlap between bins. The SNR of each feature was then calculated across all 135 channels. The SNR is a measurement used to compare the average level of the desired signal to the unwanted signal, also known as the noise. This calculation can also be visualized in Fig. 3 and 4, with the data shown in the black shaded region used as noise/rest data while data in the red shaded regions was used as signal/active data. This value can be used to assess signal quality and strength.


Figure 4: Principal Component Analysis Principal component analysis was used to analyze the discriminability between the activity recorded during different hand movements. A matrix of RMS data was input from all 135 channels. Results show RMS feature is much more differentiable in able-bodied data when compared to SCI data.
Legend Noise/Rest Region
Signal/Active Region

Figure 3: Feature Extraction Data shown comes from one channel of a single trial of recording with an able-bodied participant and a participant with SCI, respectively. Signal features used in this analysis were the root-mean-square (RMS), zero-crossings, and power. Data shown in this figure was filtered using 30 and 450 Hz cutoffs.
3.2 Signal-to-Noise Ratio in Able-bodied and Spinal Cord Injury Participants
There is a dramatic decrease in the SNR values of SCI to able-bodied participants. This trend is true across all four hand movements. The decrease in SNR was most notable in the tripod grasp, where the SCI SNR had a 118.9% difference to that of the able-bodied SNR. The trend is least notable in the hand open movement although the SCI SNR still only had an 83.1% difference to that of the able-bodied SNR. The two participants tested also had different movements yield the lowest SNR. For the participant with SCI the lowest SNR came from the tripod grasp, while the hand open movement provided the lowest SNR for the able-bodied participant (Table 1).
Movement
Cylindrical Lateral Tripod Hand Open Able-Bodied
4.8175 3.2460 4.4340 2.8665 SCI
1.3004 1.2103 1.1278 1.1839 Percent Difference (%) 115.0 91.4 118.9 83.1
Table 1: Calculated Signal-to-Noise Ratio Across Hand Movements The SNR can be calculated to assess the quality of data. Data shown in this table was filtered using 30 and 420 Hz cutoffs. A low SNR (≈1) means the data overall is noisy or the active data is not very differentiable from rest, while a high SNR (>>1) means the active data is much higher in amplitude than the corresponding rest data. When comparing the average percent difference in SNR of the two patients, signals were approximately 102.1% stronger in the able-bodied participant than the SCI participant.
4. Discussion
The poor results of the PCA and large difference in the SNR of the SCI data compared to the able-bodied data are due to the damage in the corticospinal tract after SCI. The clinically paralyzed muscle fibers are unable to produce myoelectric signals of significant strength, resulting in poor quality of data.
The poor results of the PCA can also be due to the small level of control the participant with SCI has over his hand. The participant has a very limited ability to move his fingers, resulting in no displacement when attempting to do hand movements. As the patient is therefore effectively doing the same hand motion for each different movement, it further contributes to nearly no differentiation of hand movements in the SCI EMG data.
Another factor adding to the difference in the quality of data from the individual with SCI is the different levels of fine finger control and strength needed to conduct each hand movement. For example, the tripod grasp, requires a higher degree of fine finger control, which is very difficult for the participant with SCI. In another movement, for example the hand open, the grasp requires more hand and finger strength rather than control. The individual with SCI did have relatively good strength of wrist extensor muscles. These facts most likely contributed to the varying differences in the SNR trends of the two participants.
5. Conclusion
Myoelectric signals can be recorded from participants with clinically paralyzed muscles due to SCI. After processing and analyzing signal features, the discrimination of hand movements proved to be very difficult in signals recorded from a participant with SCI when compared to able-bodied signals. Consequently, the features used in this analysis proved to be insufficient for the control of neuro-prosthetics. As our future goal continues to be to enable people with SCI to use EMG signals from affected muscles to control assistive devices, we hope to continue to refine our method of processing and filtering data. Future work will include testing other signals features so that we are able to discriminate hand movements with a higher accuracy. Accordingly, we hope to implement this method to allow for the online classification of hand movements, which will be a big step in helping improve the lives of people affected by SCI.
6. Acknowledgements
The author would like to thank Dr. Douglas Weber, the Swanson School of Engineering, and the Office of the Provost of the University of Pittsburgh for their contributions.
7. References
[1] Spinal Cord Injury Facts and Figures at a Glance. National Spinal Cord Injury Statistical Center. (2018).
[2] Ting, Jordyn, et al. “A Wearable Neural Interface for Detecting and Decoding Attempted Hand Movements in a Person with Tetraplegia.” 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2019, doi:10.1109/embc.2019.8856483.
[3] Phinyomark, Angkoon, et al. “Feature Reduction and Selection for EMG Signal Classification.” Expert Systems with Applications, vol. 39, no. 8, 2012, pp. 7420–7431., doi:10.1016/j. eswa.2012.01.102.
[4] Zardoshti-Kermani, M., et al. “EMG Feature Evaluation for Movement Control of Upper Extremity Prostheses.” IEEE Transactions on Rehabilitation Engineering, vol. 3, no. 4, 1995, pp. 324–333., doi:10.1109/86.481972.
[5] Güler, Nihal Fatma, and Sabri Koçer. “Classification of EMG Signals Using PCA and FFT.” Journal of Medical Systems, vol. 29, no. 3, 2005, pp. 241–250., doi:10.1007/s10916-005-5184-7.










