
15 minute read
Metformin administration impairs tendon wound healing
Catherine Grace P. Hobayan a, b , Arthur R. McDowell a , Feng Li a , Jianying Zhang a* , and James H-C. Wang a, b, c a MechanoBiology Laboratory, Departments of Orthopaedic Surgery, b Bioengineering, c Physical Medicine and Rehabilitation, University of Pittsburgh, PA, USA
Grace Hobayan is a fourth-year bioengineering student at the University of Pittsburgh Swanson School of Engineering. She is interested in the intersection between cellular/tissue engineering and biomechanics, and she plans to attend medical school after graduation and apply her bioengineering background to the practice of medicine. Currently, she serves as a Teaching Assistant for Intramural Internship (BIOENG 1002), where she helps bioengineering students develop presentations and effectively communicate about their research projects to a wide variety of audiences. Grace Hobayan
Dr. Jianying Zhang has interdisciplinary education background and overlapping research interests. Dr. Zhang’s research has resulted in over one hundred peerreviewed research publications and more than 35 patents, and some of the products generated from her patents have been approved by the FDA (USA) and put into clinical practice. Currently, Dr. Zhang is a research associate professor in the Department of Orthopaedic Surgery at the University of Pittsburgh School of Medicine. Dr. Jianying Zhang
Significance Statement
Obese patients with Type II diabetes often experience tendon injuries due to the exertion of higher loads on their tendons. Metformin is one of the most common medications for such prescribed for Type II diabetes. However, the effect of metformin on tendon disorders is largely unknown. This study has shown that HMGB1 is necessary for tendon healing, and the inhibition of HMGB1 by metformin impairs tendon wound healing. Thus, metformin may exacerbate healing of injured tendons in obese diabetic patients.
Category: Experimental research Keywords: Metformin, HMGB1, cell tracking, tendinopathy Abbreviations: HMGB1 - High mobility group box 1, Scx- Scleraxis, α-SMA - α-smooth muscle actin, Met - metformin, PT - Patellar tendon, AT - Achilles tendon, TSC - tendon stem cell
Abstract
Tendon injury is common, and injured tendons have a limited healing ability. High mobility group box-1 (HMGB1) has been found to enhance wound healing by recruiting cells to migrate to the wound area and increasing cell proliferation. However, the role of HMGB1 in tendon wound healing is currently unknown. Metformin, a hypoglycemic anti-inflammatory drug for Type II diabetes, inhibits HMGB1 activity by binding to its C-tail. Therefore, in this pilot study, we hypothesized that inhibiting HMGB1 by metformin slows the healing of wounded tendons due to its inhibition of HMGB1 activity. To test this hypothesis, a window defect was created in the patellar tendon and Achilles tendon of α-SMA-Ai9-Scx-GFP transgenic mice. The animals were injected either with saline every day (control group), or five days metformin before surgery (short term metformin), or metformin every day (long term metformin). Fluorescence microscopy images of tendon sections taken 4 weeks post-injury indicated that paratenon cells migrated into the wounded area of the tendon in the saline injection mice (control group), but not in the groups that received metformin injection. Cell densities in the wound area and HMGB1 serum levels were higher in the absence of metformin. This may indicate that metformin inhibited HMGB1 activity and reduced wound healing by blocking the recruitment and migration of paratenon cells to the wounded area. Thus, metformin administration limits the healing capacity for wounded Achilles tendons by limiting the migration of paratenon cells to wounded areas.
1. Introduction
Tendinopathy is a prevalent tendon disorder that affects a large proportion of people in both athletic and occupational settings [1]. However, the current treatment options for tendinopathy are largely palliative because the mechanisms causing the tendon disorder are not well understood [2]. Many intrinsic and extrinsic risk/causative factors can predispose to the development of tendinopathy. Among them, diabetes mellitus is an important risk/ causative factor [3]. Obese patients with Type II diabetes often experience tendinopathy due to the exertion of higher loads on their tendons.
Metformin is a hypoglycemic anti-inflammatory drug commonly used for treatment of Type II diabetes. High mobility group box 1 (HMGB1) is an alarmin protein released from necrotic cells to induce inflammatory responses in the human body. Extracellular HMGB1 induces several responses, including the activation of proinflammatory cytokines, cell proliferation and stromal cell matrix responses [4]. Metformin has been shown to bind to the acidic tail of HMGB1 and inhibit its inflammatory activity in a concentration-dependent manner [5]. The inflammatory activity of HMGB1 has also been shown to contribute to tendon injury mechanisms by regulating inflammatory cytokines and matrix changes [6]. No prior studies have analyzed the effects of metformin in the context of tendinopathy.
Typically, tendinopathy is studied using the samples from the patients who choose surgical interventions to alleviate tendinopathy symptoms. The limitations of the sample size and source have made it difficult to devise an effective treatment modality for the tendon disease. Thus, animal models of tendinopathy are required to investigate the cellular and molecular mechanisms regulating the tendon disorder to devise effective treatment protocols. Moreover, to develop better treatment options for tendinopathy, it is essential to have a reproducible, cost-effective animal model of tendinopathy that will allow the evaluation of the tendon disease progression and enable the development of better treatment options.
Tendon stem/progenitor cells (TSCs) are essential for the maintenance and repair of tendinous tissues when injured. Scleraxis (Scx) and α-smooth muscle actin (α-SMA) are the markers of tendon and mesenchymal progenitor cells, respectively, which are suggested to be involved in tendon wound healing [7]. However, little is known about the anatomical origin of these resident progenitors within the tendon that contribute to natural healing following injury.
In this pilot study, we used α-SMA-Ai9-Scx-GFP transgenic mice that express both Scx and α-SMA to characterize the expansion of resident tendon progenitors that contribute to natural wound healing during adulthood and investigate the effect of HMGB1 on wounded tendon healing by inhibiting the activity of HMGB1 with metformin injection. We hypothesized that metformin would reduce the extent to which wounded tendons are healed because of its inhibitory function on HMGB1inflammatory activity. We also hypothesized that administration of metformin both before and after tendon injury would reduce healing capacity to a greater extent than only administering metformin before injury. This information will be beneficial for obese patients with Type II diabetes who regularly take metformin and are afflicted with tendinopathy.
2. Methods 2.1 Transgenic mice
Tamoxifen-inducible α-SMA-CreERT2 mice were crossed with Scx-GFP mice, and then crossed with Ai9-Cre reporter mice to generate triple transgenic SMA-Ai9-ScxGFP mice. This allowed for tenocytes and paratenon cells to be visualized via fluorescence microscopy [7]. 2.2 Wound healing model
Three intraperitoneal injections of tamoxifen (Sigma Aldrich, St. Louis, MO) were delivered on consecutive days to 2-month-old SMA-Ai9-ScxGFP mice at a dose of 100 μL of 20 mg/mL prior to patellar tendon (PT) injury by 1 mm diameter biopsy punch (Miltex, Inc., York, PA) and Achilles tendon (AT) injury using a 0.5 mm punch (Word Precision Instruments, Sarasota, FL). The animals were divided into three groups (6 mice per group): Group 1 (Saline) received intraperitoneal (IP) injections with saline every day; Group 2 (Met-Short term) received IP injections with Met (160 mg/kg/ day) for 5 days before surgery; Group 3 (Met-Long term) received IP injections with the same dose of Met for 5 weeks (5 days before surgery and 4 weeks after surgery). For the Met-Short and MetLong groups, the same dosage of metformin was administered, but the frequency of administration is different. Met-Short involves only pre-surgery injection, and Met-Long involves both pre- and post-surgery injections to investigate whether higher frequency of metformin leads to further inhibition of tendon wound healing mechanisms. All mice were sacrificed at day 30 post-injury, their blood was collected, and both PT and AT of each mouse was harvested. The potential effect of HMGB1 on wounded tendon healing and cell migration was assessed by histological analysis. 2.3 HMGB1 determination in mouse serum
The HMGB1 levels in serum of the mice at 30 days postsurgery were determined using an ELISA kit according to the manufacturer’s protocol (Shino-Test Corporation, Tokyo, Japan). Briefly, 0.5 mL of blood samples was collected from the heart of each mouse immediately after they were sacrificed, and the blood samples were stored at room temperature for 1 hour. The serum was separated by a centrifuge at 2000 g for 30 min. The supernatants were used for the measurement of HMGB1. All samples were analyzed from 3-6 mice in each group (n=3-6). 2.4 Histological analysis
At 30 days after surgery, the tissue samples were harvested and placed in pre-labeled base molds filled with frozen section medium (Neg 50; Richard-Allan Scientific, Kalamazoo, MI). The base mold with tissue samples was immersed in liquid nitrogen cold 2-methylbutane and allowed to solidify completely. The tissue blocks were either stored in a deep freezer (-80ºC) or cut into 5 µm thick sections for histological analysis. The tissue sections were placed on glass slides and allowed to dry overnight at room temperature. The cell migration and the wounded tendon healing were analyzed under a fluorescent microscope (Nikon eclipse, TE2000-U) using SPOT imaging software (Diagnostic Instruments, Inc., Sterling Heights, MI). 2.5 Semi-quantification of the extent of cell migration The healing results were further analyzed by semiquantification on tendon tissue sections. Briefly, four views from each tendon were randomly chosen on a microscope with a magnification of 20 ×. Then, the Scx-GFP positive (green fluorescence) cells and α-SMA-Ai9 positive (red fluorescence) cells were identified manually and computed by SPOT IMAGING Software. Next, the proportion of Scx-GFP in total cells was calculated by dividing the green cell numbers by total cell numbers, or the sum of the green and red cell numbers. Similarly, the proportion of α-SMA-Ai9 in total cells were calculated by dividing the red cell numbers by total cell numbers. Twelve ratio values (four views/section × 3 sections/mouse) were averaged to obtain the percentage of Scx-GFP positive or α-SMA-Ai9 positive cells, which represents the extent of cell migration in the respective wound area.
2.6 Data analysis
The results presented in the figures are representative of these (mean ± SD, n = 3 to 6). Two-tailed student t-test (type=3) was used for statistical analysis to compare the cell numbers and serum levels of HMGB1 between Saline and Met-Short; Saline and Met-Long; and Met-Short and Met-Long groups. A p-value less than 0.05 was considered to be significantly different.
3. Results
Gross-inspection of the wound area showed that metformin injection inhibited wound healing in both PT and AT as evidenced by unhealed wound area size and color (Fig. 1A-F). The concentration of HMGB1 in sera of Met-injected mice was significantly lower than saline injection group (Fig. 1G). The histological analysis on the frozen sections of mouse tendons showed that Met injection inhibited the cell migration in both wounded PT (Fig. 2) and AT (Fig. 3). Furthermore, saline treated mice had two times more α-SMA-Ai9 cells (red) migrated to the wound area than Met-Long term group (Figs 2, 3). Met injection decreased the migration of both Scx-GFP (green) and α-SMA-Ai9 cells (red) (Figs. 2, 3). Semi-quantification confirmed the decrease in cell migration in the wound area of PT due to long term Met treatment (Fig. 2J). Finally, Met injection decreased the serum levels of HMGB1 (Fig. 4).

Figure 1: Met injection inhibits wounded mouse tendon healing in a dosage-dependent manner. A-C: Gross view of wounded mouse patellar tendon (PT) at week-4 post-surgery; D-F: Gross view of wounded mouse Achilles tendon (AT) at week-4 post-surgery. Metformin injection decreases healing speed at a dosage-dependent manner as evidenced by wound area size (A-C) and color (D-F).
Figure 2: Metformin injection inhibits wounded patellar tendon healing by decreasing tendon cell migration. A-C: Scx-GFP cells; D-F: α-SMA-Ai9 cells; G-I: Merged images of A-C and D-F. Both green and red cell numbers are increased in the wound area of saline injection mice (A, D, G). However, reduced cell numbers and large empty areas are found in Met injections wounds (B, E, H, C, F, I). Semi-quantification shows that the cell numbers of α-SMA-Ai9 (red) in saline group are 2 times more than that in long-term Met injection wound area (J). *p<0.05 compared to saline group; #p<0.05 compared to Met-short group.
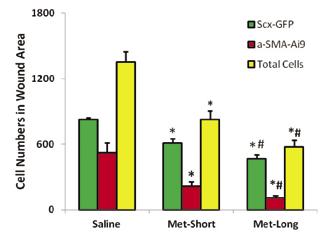

Figure 3: Met injection inhibits wounded Achilles tendon healing by decreasing tendon cell migration. A-C: Scx-GFP cells; D-F: α-SMA-Ai9 cells; G-I: Merged images of A-C and D-F; J-L: Enlarged box areas in the images of G-I.
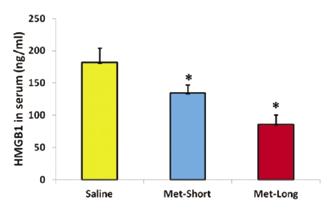
Figure 4: Met injection decreases HMGB1 levels in serum. *p<0.05 compared to saline group.
4. Discussion
This study has directly demonstrated that Met was able to block HMGB1 release as evidenced by reduced levels of HMGB1 in the sera of wounded mice. In addition, Met injection to wounded mice impaired the healing as evidenced by low cell density and large, premature unhealed wound areas found in Met treated groups. These results showed that HMGB1 plays a critical role in wound healing by recruiting α-SMA positive cells in the paratenon to the wound area. Our results also indicate that metformin has inhibited the activity of HMGB1 and prevented proper wound healing by blocking the recruitment and migration of paratenon cells to the wounded area. This is consistent with the aforementioned literature in that the presence of metformin inhibited inflammatory activity that may have contributed to proper tendon wound healing [5].
Tissue regeneration is a well-orchestrated process that occurs after injury. Understanding the molecular events underlying the regeneration process and developing agents that aid regeneration is essential for patients with injured tissue in a variety of clinical settings [8]. It has been reported that HMGB1 is essential for life because HMGB1 knockout mice die perinatal [9]. Recent studies have shown that HMGB1 induces cell migration [4, 10] and promotes muscle regeneration after acute muscle injury [8]. These findings were further confirmed by our results.
We have demonstrated that the metformin administration before tendon injury also inhibits the migration of paratenon cells to the wounded area of an Achilles tendon, and long-term administration of metformin (before and after surgery) slows down the healing. These results suggest that patients with Type II diabetes should stop taking metformin after sustaining tendon injuries.
This study provided a useful animal model for tendon wound healing. Our results have demonstrated that both Scx + resident progenitors and α-SMA + progenitors are the main contributors to natural wound healing during adulthood. One limitation of this study is that proliferation of cells in the wound area was not determined since only a single early time point was analyzed (4 weeks postinjury) over the course of the tendon healing process; only the migration of the cells could be determined. Future studies should incorporate longer time points (e.g., 8-12 weeks after surgery). Hematoxylin & eosin (H&E) staining should be used to analyze alterations in tissue structure of wounded tendons in response to metformin.
Immunohistochemistry should also be used to determine the activity of HMGB1 in future studies. Immunostaining and cell tracking should be used to determine both cell proliferation and migration at later time points in the tendon healing process. Furthermore, similar studies should be done to analyze the effects of metformin in female mice, as well as mice of different age groups.
5. Conclusion
We have demonstrated that the metformin administration inhibits the migration of paratenon cells to the wounded area of
patellar and Achilles tendons and impairs their healing via the inhibition of HMGB1 activity. Future studies are to investigate for similar effects on the tendons of female mice, measure cell proliferation, and verify tissue structure changes over multiple longer time periods to account for the slow healing process for tendons.
6. Acknowledgements
This work was supported in part by the NIH under award numbers AR061395, AR065949, and AR070340 (JHW). We thank Dr. Kelly Williamson, the Division of Laboratory Animal Resources (DLAR), and the Swanson School of Engineering (SSOE) Undergraduate Summer Research Internship for their support on this project.
7. References
[1] Scott A, Ashe MC: Common tendinopathies in the upper and lower extremities. Curr Sports Med Rep 2006, 5(5):233-241. [2] Abate M, Silbernagel KG, Siljeholm C, Di Iorio A, De Amicis D, Salini V, Werner S, Paganelli R: Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther 2009, 11(3):235. [3] Lui PPY: Tendinopathy in diabetes mellitus patientsEpidemiology, pathogenesis, and management. Scand J Med Sci Sports 2017, 27(8):776-787. [4] Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A et al: HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 2012, 209(3):551-563. [5] Horiuchi T, Sakata N, Narumi Y, Kimura T, Hayashi T, Nagano K, Liu K, Nishibori M, Tsukita S, Yamada T et al: Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. J Biol Chem 2017, 292(20):8436-8446. [6] Akbar M, Gilchrist DS, Kitson SM, Nelis B, Crowe LAN, Garcia-Melchor E, Reilly JH, Kerr SC, Murrell GAC, McInnes IB et al: Targeting danger molecules in tendinopathy: the HMGB1/ TLR4 axis. RMD Open 2017, 3(2):e000456. [7] Dyment NA, Hagiwara Y, Matthews BG, Li Y, Kalajzic I, Rowe DW: Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One 2014, 9(4):e96113. [8] Tirone M, Tran NL, Ceriotti C, Gorzanelli A, Canepari M, Bottinelli R, Raucci A, Di Maggio S, Santiago C, Mellado M et al: High mobility group box 1 orchestrates tissue regeneration via CXCR4. J Exp Med 2018, 215(1):303-318. [9] Calogero S, Grassi F, Aguzzi A, Voigtlander T, Ferrier P, Ferrari S, Bianchi ME: The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet 1999, 22(3):276-280. [10] Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L et al: Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med 2012, 209(9):1519-1528.










