
8 minute read
Robust osteogenesis of mesenchymal stem cells in 3D bioactive hydrogel
Robust osteogenesis of mesenchymal stem cells in 3D bioactive hydrogel nanocomposites reinforced with graphene nanomaterials
Eileen Li a, b , Zhong Li b , Colin Del Duke b , Hang Lin a, b, a Departments of Bioengineering, b Department of Orthopedic Surgery, Center for Cellular and Molecular Engineering, University of Pittsburgh School of Medicine, PA, USA
Eileen Li
Eileen Li is a junior bioengineering student who is currently pursuing the cellular and biomechanical engineering tracks. She has been working with Dr. Lin at the Center for Cellular and Molecular Engineering of the Department of Orthopaedic Surgery for 2 years. Her research interests focus on developing biomaterial scaffolds for bone regeneration and tissue engineering.
Dr. Lin is an assistant professor (tenure track) working in the Department of Orthopaedic Surgery. His research interests are to understand the relationship between aging and osteoarthritis (OA), develop disease modifying drugs to treat OA, and regenerate articular cartilage through tissue engineering strategy. Currently, Dr. Lin is supported by both internal and external grants, including several ones from the NIH. Dr. Hang Lin
Significance Statement
Treatment of bone defects is presently limited by the insufficient number of suitable bone grafts. A possible solution to this is the use of nanomaterial incorporated bioactive hydrogels to develop biosynthetic bone grafts. Our work demonstrates that the novel 2D nanomaterial, silica-coated graphene oxide (SiGO), can promote the osteogenic differentiation of human mesenchymal stem cells in 3D hydrogels and therefore holds promise in bone tissue engineering.
Category: Experimental research Keywords: hydrogel, osteogenic differentiation, nanomaterials, silica-coated graphene oxide Abbreviations: silica-coated graphene oxide (SiGO), graphene oxide (GO), mesenchymal stem cells (MSC), methacrylated gelatin (gelMA)
Abstract
A major challenge facing bone defect treatment is the limited availability of functional, natural bone grafts. To combat this issue, bone grafts engineered from stem cells and synthetic bioactive materials are attracting attention as an alternative approach to bone defect treatment. Silica-coated graphene oxide (SiGO) can be potentially used for the development of engineered bone grafts as silicon (Si) is essential for bone remodeling and growth. SiGO nanosheets were combined with methacrylated gelatin (SiGO/ GelMA) and used to resuspend MSCs. The solution was then photocrosslinked to create 3D cell containing scaffolds. . Usually, osteogenic growth factors are used to enhance osteogenic differentiation during cell culture, however there is the possibility that SiGO can enhance differentiation without the addition of these growth factors. The GelMA and SiGO/GelMA scaffolds were cultured in osteogenic medium for 4 weeks with no supplement of osteogenic growth factors The viability of cells encapsulated in the scaffolds were unaffected by SiGO addition. The expression levels of major osteogenic marker genes were generally higher in the SiGO/GelMA group than GO/GelMA. Calcein green staining, histology and immunohistochemistry results all indicated significantly more homogeneous and robust calcification in the SiGO/GelMA scaffolds. The results suggest that SiGO may hold immense potential in MSC-based bone tissue engineering and regeneration.
1. Introduction
Large bone defects, fracture-delayed unions, and non-unions have become more prevalent. A major issue for the treatment of bone injury is obtaining functional bone grafts for repair. Currently, autografts, allografts and xenografts are widely used clinically for bone defect management. Autografts remain the “gold standard” treatment, but have very limited availability. This method basically creates another area of injury for the patient and allows for the chance of many more complications to occur [1]. Allografts and xenografts are harvested from deceased donor and animals, respectively, thus making availability less of an issue. However, they pose high risks of disease transmission and immunological rejection [1]. To combat this, the applications of bone grafts engineered from stem cells and synthetic, bioactive materials are attracting more and more attention. Nanomaterials, with their unique physical and chemical characteristics, have been used in a broad array of biomedical applications. It has been reported that certain nanomaterials can help promote protein absorption and trigger signaling pathways, which may be exploited to direct cell behavior [3]. For example, if used appropriately, nanomaterials may assist in upregulating osteogenic differentiation of mesenchymal stem cells (MSCs) for creating tissue engineered bone, therefore eliminating the need and complications associated with the use of autografts, allografts or xenografts. Recent studies have utilized 2D graphene nanomaterials and their derivatives such as graphene oxide (GO) in the hope that these nanomaterials can provide satisfactory mechanical and biological environments for stem cell-based bone tissue engineering. Silica-coated graphene oxide
(SiGO) is of particular interest in bone tissue engineering as silicon (Si) is a key trace element that has been reported to be essential for maintaining healthy bone and promote bone remodeling [4]. In this research, we hypothesize that the incorporation of SiGO nanosheets in 3D hydrogel scaffolds can significantly enhance the osteogenic differentiation of human MSCs for potential bone repair and regeneration applications.
2. Methods
With IRB approval (University of Washington), MSCs were isolated from femoral heads and trabecular bone of human patients undergoing total knee arthroplasty. GO was synthesized using a modified Hummers method and then converted to SiGO nanosheets via a sol-gel method reported in our previous study [2]. The SiGO nanosheets were combined with 15% methacrylated gelatin (SiGO/GelMA) at 1mg/mL. This composite solution was used to resuspend MSCs at 20M cells/mL and photocrosslinked using 395 nm UV to create 3D cell-laden scaffolds. The scaffolds were cultured in osteogenic media (DMEM with 10% FBS, 1% AntibioticAntimycotic, 10 nM dexamethasone, 0.1 mM L-ascorbic acid 2-phosphate, and 10 mM beta-glycerophosphate, 10 nM vitamin D3) for 4 weeks with no supplement of osteogenic growth factors. The cytocompatibility of SiGO/GelMA was analyzed using the Live/ Dead cell viability assay. Real-time polymerase chain reaction (RT-PCR) was performed to analyze osteogenic gene expression. Histological staining and immunohistochemistry (IHC) were used to further assess osteogenesis quality. Pure GelMA scaffolds were prepared and cultured under identical conditions for comparative purposes.
3. Results
The viability of cells encapsulated in the scaffolds were unaffected by SiGO addition, as proved by Live/Dead staining (Figure 1). The expression levels of major osteogenic marker genes, including osteocalcin (OCN) and bone morphogenetic protein 2 (BMP2) (Figure 2), were quantified with reverse transcriptase polymerase chain reaction (RT-PCR) and generally the highest expression of these proteins were in the SiGO/GelMA group. Alizarin red staining, Von Kossa staining and Calcein Green staining all indicated significantly more homogeneous and robust MSC calcification in SiGO/GelMA scaffolds than in the other groups (Figure 3 - 4). Through Immunohistochemistry staining, the highest amount of alkaline phosphatase (ALP) and OCN was identified in the SiGO scaffolds (Figure 5).
Figure 1: Live/Dead staining: (A) GelMA, (B) SiGO/GelMA. After 4 day culture, SiGO showed no negative effect on the viability of MSC’s and is therefore biocompatible (scale bar = 200µm)
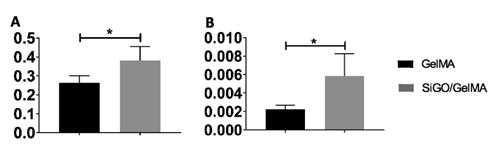
Figure 2: Expression of osteogenic markers: A) OCN, B) BMP2. RT-PCR results indicate that SiGO/GelMA scaffolds led to higher expression of OCN and BMP2 in the cells than the GelMA scaffolds. (* P<0.05)

Figure 3: Alizarin red staining: (A) GelMA, (B) SiGO/GelMA. The SiGO/GelMA scaffolds had significantly more calcium deposition after 4 weeks of osteogenic differentiation (scale bar = 1mm)
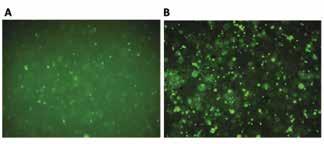
Figure 4: Calcein Green fluorescent imaging: A) GelMA, B) SiGO/GelMA. SiGO containing scaffolds showed more calcein green staining after 4 week culture, indicating more calcium minerals than the GelMA scaffolds

Figure 5: Immunohistochemistry staining: A) ALP staining of GelMA, B) OCN staining of GelMA, C) ALP staining of SiGO/GelMA, D) OCN staining of SiGO/ GelMA. After 4 week cell culture, the SiGO/GelMA scaffolds showed significantly more ALP and OCN than the GelMA ones (scale bar = 100 µm)
4. Discussion
The high expression levels of major osteogenic marker genes for the scaffolds indicate that the MSC’s have undergone osteogenic differentiation. These marker genes were expressed at higher levels for SiGO-containing scaffolds, suggesting that SiGO is more osteoinductive, induces osteogenesis, than unmodified GO. Furthermore, the Alizarin red staining and Von Kossa staining, which indicate calcium and phosphate deposition, respectively, show more robust and homogenous mineralization in the SiGO group. Higher calcium and phosphate deposition is a good indicator of the presence of osteogenically differentiated MSC’s. This is further supported by the fluorescent calcein green staining, which demonstrates the homogeneous 3D distribution of calcium minerals throughout the SiGO/GelMA scaffolds in a larger quantity than in pure GelMA. This suggests that the scaffold biomaterial has an effect on transporting certain proteins that assist in upregulation of osteogenesis in the MSCs, which warrant further investigation. It is worth noting that the SiGO nanomaterial did not elicit any adverse effect on cell viability, as proved by the live/dead assay. Overall, SiGO provides a robust environment for the differentiation of MSC’s into bone tissue.
5. Conclusion
The results suggest that in comparison to other nanomaterials, SiGO may hold immense potential in MSCbased bone tissue engineering and regeneration. We believe the mechanically strong core and biologically active shell of SiGO nanoplatelets synergistically promote osteogenic differentiation. For future research, mechanical testing will be performed on the scaffolds to quantify their compressive modulus and western blot will be utilized to decipher the molecular mechanisms underlying the osteo-inductive properties of SiGO.
6. Acknowledgements
Summer Research Internship (E.L.) funded by the Swanson School of Engineering and the Office of the Provost. This research is in part supported by the National Institutes of Health (NIH UG3TR002136).
7. References
[1] Aitken G, et al. Benefits and associated risks of using allografts, autograft and synthetic bone fusion material for patients and service providers. JBI Database of Systematic Reviews and Implementation Reports 8(8), 1-13, 2010. [2] Li Z, et al. Incorporating Silica-coated Graphene in Bioceramic Nanocomposites to Simultaneously Enhance Mechanical and Biological Performance. J Biomed Mater Res A, 2020, in press.
[3] McMahon R, et al. Development of nanomaterials for bone repair and regeneration. J Biomed Mater Res 101B(2), 387-397, 2013.
[4] Gotz W, et al. Effects of Silicon compounds of biomineralization, osteogenesis, and hard tissue formation. Pharmaceutics 11(3) 117-144, 2019.










