
9 minute read
techniques for the treatment of dry eye disease
Manufacturing a polyelectrolyte coating on contact lenses using automated vs. manual techniques for the treatment of dry eye disease
Zixie Liang a , Alexis Nolfi a, b , and Bryan Brown a, b a University of Pittsburgh, b McGowan Institute for Regenerative Medicine, Pittsburgh, PA
Zixie Liang
Zixie Liang is a Senior Bioengineering Student at the University of Pittsburgh with a cellular concentration and a chemistry minor. She is also an Undergraduate Research Assistant at McGowan Institute for Regenerative Medicine.
Dr. Bryan Brown is an Associate Professor in the Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute at the University of Pittsburgh. He is also a core faculty member of the McGowan Institute for Regenerative Medicine where he serves as the Director of Educational Outreach. Dr. Brown is also an Adjunct Assistant Professor of Clinical Sciences at the Cornell University College of Veterinary Medicine and Chief Technology Officer of Renerva, LLC, a Pittsburgh-based start-up company. Dr. Bryan Brown
Significance Statement
Dry eye disease (DED) is a common ocular disease worldwide and is characterized by inflammation mediated by proinflammatory macrophages, however, treatment currently cannot provide an effective and sustained relief. This project aims to establish a new treatment for DED that creates a nanometer thick coating on contact lenses with a drug delivery system that releases immune modifying drugs efficiently to patients’ eyes. This immune modifying drug is capable of shifting macrophage phenotype, therefore reducing inflammation. The automated and manual manufacturing techniques to coat lenses are compared in this paper.
Category: Experimental research Keywords: Layer-by-layer coating, macrophage, contact lenses, dry eye disease
Abstract
Dry eye disease (DED) is a multifactorial disease associated with a diminished quality of tear film and compromised ocular surface. It is recognized that inflammation mediated by macrophages is critical to the development of DED; therefore, we propose to coat an immune modifying drug that shifts macrophages from a pro-inflammatory (M1) to anti-inflammatory (M2) phenotype on to contact lenses with a polymer delivery system. This should provide for a sustained release of drug over time. Drug coated lenses were made either via automated coating method using a machine or in a manual way and then compared. Alcian blue staining and drug release kinetics show that immune modifying drug can be successfully coated on to lenses, and manually made lenses tend to be more uniformly coated but release less drug over time. The results of this study demonstrate that combination of current automated and manual methods should be used for the next-stage lens manufacturing for better drug coating and efficiency.
1. Introduction
Dry eye disease (DED) is a prevalent disease in the US and worldwide affecting millions of people, especially middle-aged and over [1]. Patients with dry eye disease often experience visual disturbances, eye dryness, irritation, and light sensitivity, which decreases the quality of life [2]. Treatments such as over the counter and prescription eye drops only provide transient and temporary relief and do not change the underlying disease [3]. Therefore, a new treatment for DED is desired.
Past research revealed that DED is a disease with a core mechanism of inflammation and that this inflammation is mediated by macrophages [4]. A treatment for DED may be achieved by manipulating the polarization of macrophages to shift from a proinflammatory (M1) to an anti-inflammatory (M2) phenotype [4]. Previous studies focusing on polypropylene mesh have shown that immunomodulatory cytokines that promote an M2 phenotype can be released from a nanometer-thickness polymer coating loaded onto the surface of a biomaterial implant [5]. Therefore, we propose to use this immune-modifying drug with a polymer delivery system to create a coating on a contact lens. We hypothesize that this will give a sustained release of drug over time to remedy the defect of current DED treatment.
To manufacture these coated lenses, we either produced them using a machine in an automated way or produced them manually by hand. The machine coated vs. hand coated method was compared during this experiment.
Figure 1: Procedure of layer-by-layer coating performed using chitosan as polycation and dermatan sulfate as polyanion in cycles.
2. Methods 2.1 Layer-by layer coating
A layer-by-layer procedure was conducted in order to deposit a uniform coating capable of releasing immune-modifying drugs. Chitosan (2mg/mL) was used as the polycation and dermatan sulfate (2mg/mL) was used as the polyanion in order to build up base layers (Fig.1).
For manually produced lenses, lenses were placed in a cage and moved by hand between polymer solutions and washes (Fig.2). Residual polymer was tapped off in-between steps. For lenses made in an automated way, lenses were secured between tweezers and clamped into a Silar Controller (MTI Corporation, Richmond, CA) automated staining apparatus where the machine moved lenses between solutions and washes (Fig.3).

Figure 2: Set up of hand dipping method (i), and the lens cage (ii) used to secure lens.

After 10 cycles of non-drug-containing base polymer layers were added, a mixture of immune modifying drug (1.5µg/mL) and dermatan sulfate (2mg/mL), along with chitosan, was used to build up another 40 drug-containing layers on the lenses. Uncoated lenses were used as a control. 2.2 Coating characterization—Alcian blue staining
Alcian blue, a blue stain that stains glycosaminoglycan components, staining was performed to confirm lenses had been successfully coated with polymers in a uniform and conformal way. Hand coated lens, machine coated lens, and uncoated lens were re-hydrated in distilled water and submerged into Alcian blue dye for 30 minutes at room temperature, and then the lenses were washed in running distilled water for 5 min. Stain was observed and captured by camera. 2.3 Drug cumulative release and kinetics—Controlled drug release assay with ELISA assay
A controlled drug release assay and subsequent ELISA assay were performed to test if the drug was able to be released in a relatively more sustained way and if there were differences in release amount or kinetics due to differences in manufacturing. Immune modifying drug coated lenses (using manual and automated methods, n of 1 per condition) and uncoated lenses were immersed into a 500 μL solution of 1x PBS containing the enzymes chitosanase (0.05units/mL) and chondroitinase ABC (0.05 units/mL). Lenses were then incubated at 37°C with agitation. The solution was collected and changed every 24 hours in the first week, and then every 48 hours in the following 2 weeks. ELISA was then run using a commercially available kit (R&D Systems, Minneapolis, MN) in order to assess amount of drug release at each timepoint, followed by the generation of a graph showing cumulative release of drug over time.
3. Results
Images of Alcian blue stained lenses were captured and shown below in Figure 4. Both the hand dipped lens (i) and machine dipped lens (iii) were stained blue while the uncoated control lens (ii) remained clear. This experiment was repeated twice with a total of 2 lenses per condition. The hand dipped lens
appeared to be more uniformly and consistently stained, while the machine dipped lens had an unstained portion on the top where it was clamped to the machine. At the same time, the machine dipped lens showed a darker staining than the hand dipped lens.
Figure 4: Alcian blue staining of hand dipped (i), uncoated control (ii), and machine dipped (iii) lenses. Color blue indicate successful coating.

From ELISA, a graph of cumulative release of drug over time was made and shown in Figure 5. For both coating methods, the amount of drug released increased rapidly in the first 50 hours and reached its maximum at around 100 hours. However, the machine dipped method produced lenses that released approximately 2000 pg more immune modifying drug than the hand dipped method. The uncoated control lens shows no release of drug.
Figure 5: Cumulative release of drug comparing lenses produced in an automated way (red circles) and lenses produced in a manual way (blue circles). Uncoated control lenses (green circles) show no release of drug.
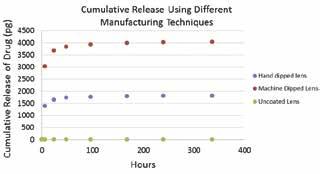
4. Discussion
Blue stain on the hand dipped and machine dipped lenses indicate that both the manual and automated coating method can successfully load the coating on to the lens. The unstained portion on the machine dipped lens shows that the machine dipped method is less uniform due to the tweezer used for attachment to the machine. The darker staining on the machine dipped lens suggests a larger amount of polymer deposition. This result also corresponds with the cumulative release over time analysis that shows that manually dipped lenses, while having similar release kinetics, tend to release less drug overall than lenses dipped in an automated way. This could be attributed to the more thorough polymer removal and drying between steps in the manual method. Overall, the manual method showed a better ability of coating; however, the automated method is more efficient and convenient. Future study will try to incorporate the cage concept we used for manually coated lenses into the automated method to avoid direct contact from tweezer to lenses, which should help to make the coating more uniform. This combination could also make the manufacturing process more efficient. To achieve this goal, we would design a 3D printed cage that can be secured onto the machine and is able to hold several lenses at the same time. More experiments investigating the amount and release time of immunemodifying drugs from contact lenses that can be manufactured using numerous techniques would be also conducted in order to identify the best technique for project scale-up. Future studies also include in vitro and in vivo experiments to confirm maintenance of drug bioactivity and efficacy in dry eye animal models.
5. Conclusion
This investigation demonstrated that a uniform coating consisting of chitosan and dermatan sulfate can be coated onto contact lenses in order to deliver immune-modifying drugs and that this coating is more uniformly applied when using a nonautomated manufacturing technique. The length of release is the same for both automated and non-automated methods, but the automated produced lens released more immune modifying drug than the non-automated lens. Future studies will determine the best technique of coating to prepare the in vitro and in vivo study of the contact lenses.
6. References
[1]. DA Schaumberg, DA Sullivan, et al. August 2003. Prevalence of dry eye syndrome among US women. Am J Ophthalmol 136(2):318–326.
[2]. W Stevenson, SK Chauhan, et al. January 2010. Dry eye disease: an immune-mediated ocular surface disorder.Arch Ophthalmol 130(1):90-100.
[3]. 2007. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf 5(2):75–92.
[4]. I You, T Coursey, et al. August 2015. Macrophage Phenotype in the Ocular Surface of Experimental Murine Dry Eye Disease. Arch Immunol Ther Exp (Warsz) 63(4): 299-304.
[5]. D Hachim, S LoPresti, et al. October 2016. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Elsevier Biomaterials 112(2017): 95-107.
7. Acknowledgments
Study conducted at the McGowan Institute for Regenerative Medicine in Dr. Bryan Brown’s Lab. Many thanks to Alexis Nolfi for guiding the experiments and for being an excellent mentor.










