15 minute read
Liam Martin, Megan R. Routzong, Ghazaleh Rostaminia, Pamela A. Moalli, Steven D. Abramowitch
Changes to the maternal sacrum and coccyx during and after pregnancy and delivery
Liam Martin a , Megan R. Routzong, BS a , Ghazaleh Rostaminia, MD, MSc b , Pamela A. Moalli, MD, PhD c , Steven D. Abramowitch, PhD a a Translational Biomechanics Laboratory, Department of Bioengineering, Swanson School of Engineering, University of Pittsburgh, Pittsburgh, PA, USA b Female Pelvic Medicine and Reconstructive Surgery (PFMRS), Division of Urogynecology, University of Chicago Pritzker School of Medicine, Northshore University HealthSystem, Skokie, IL, USA c Department of Obstetrics, Gynecology & Reproductive Surgery, University of Pittsburgh, Pittsburgh, PA, USA; Magee-Womens Research Institute, Pittsburgh, PA, USA
Liam Martin
Liam Martin is a senior bioengineering student on the biomechanics track with a minor in mechanical engineering. He has worked for ten months in the Translational Biomechanics Laboratory where he has worked to help describe the effects of pregnancy and delivery on the maternal bony pelvis.
Dr. Abramowitch received his B.S. (1998) in Applied Mathematics and Ph.D. (2004) in Bioengineering from the University of Pittsburgh. Currently, he is an Associate Professor in the Department of Bioengineering and serves as the Director of the Translational Biomechanics Laboratory. This past October he was the recipient of the Biomedical Engineering Society (BMES)diversity lecture award at the national conference in Philadelphia. Dr. Abramowitch’s research focuses on understanding the impact of pregnancy, delivery, and other life events (aging, menopause, etc.) on the structural integrity of the pelvic floor in women. Dr. Steven D. Abramowitch
Significance Statement
Changes to the maternal pelvis during pregnancy and after delivery have yet to be robustly quantified, but could eventually allow for identification of women at risk of sustaining injury during vaginal delivery. By looking at the combined maternal sacrum-coccyx shape, we found significant posterior movement of the coccyx with respect to the sacrum during pregnancy and, in some women, after delivery.
Category: Computational research Keywords: Delivery, Pregnancy, Maternal bony pelvis
Abstract
Hormonal changes during pregnancy cause tissue remodeling, presumably to facilitate vaginal delivery. This study aimed to determine whether softening of maternal tissues results in sacrumcoccyx shape changes by comparing measurements between nulliparous (have never given birth), gravid (pregnant), and parous (have given birth) women. We hypothesized that these measures would differ significantly between groups and be consistent with remodeling that would facilitate vaginal delivery (i.e. posterior movement of the coccyx to accommodate the fetus). Assuming that some women do not fully recover from delivery, we expect to see differences between all three groups. Sacrum and coccyx features were measured by analyzing pelvic MRI scans. Of the 12 measures performed, 3 had significant univariate results: coccygeal curvature index (p<0.001), sacrococcygeal curvature index (p<0.001), and sacrococcygeal angle (p=0.010). Only the nulliparous and gravid groups differed significantly, while the parous values straddled both groups. The results of this study support the hypothesis that pregnancy results in significant changes to the combined maternal sacrum/coccyx shape that are consistent with those more favorable for vaginal delivery and implies that lasting changes occur during pregnancy and/or delivery. Additionally, when dividing these groups into subgroups defined by parity (number of deliveries), larger shape changes were quantified with increasing parity in the gravid group. Because our gravid patients had yet to give birth vaginally (vaginally nulliparous), these changes are likely due to pregnancy alone as a C-section is not expected to affect pelvic shape. This suggests that pregnancy, despite mode of delivery, can result in unrecoverable pelvic shape changes.
1. Introduction
Hormonal changes during pregnancy are known to cause tissue remodeling, resulting in connective tissue laxity at the pubic symphysis and sacroiliac joints presumably to facilitate vaginal delivery [1]. The tissue laxity and remodeling allow for the maternal pelvis to accommodate the growing fetus. Previous work by our lab demonstrates the need for this tissue laxity as the sacrum and coccyx significantly engaged with the fetal head during simulations of vaginal delivery: the mechanical load introduced by the fetal head pushed the coccyx posteriorly, forcing the muscles and connective tissues anchored and engaged with these bones to stretch [2]. This suggests three potential sources for persistent pelvic shape changes—increases in intraabdominal pressure due to the growing fetus, tissue remodeling during pregnancy, and/or injury during vaginal delivery. If the coccyx is moved during delivery, it is reasonable to assume that tissue remodeling during pregnancy may make this motion easier. Other studies have shown significant movement of maternal bony structures during pregnancy, though sacrum and coccyx shape specifically have yet to be investigated [3,4]. Tissue remodeling along with mechanical strain from the fetus have been found to cause lower spine and pubic symphysis pain that can persist after pregnancy and delivery [5]. Also, there are other studies that show that there are many changes that occur on the pelvic floor muscles [6]. It is
then believed that this pain may be less severe in women whose pelvises require less adaptation during pregnancy and delivery. If the pelvic shape does change significantly, can we quantity it, and does it return to “normal” afterwards?
Thus, we aimed to determine whether tissue remodeling during pregnancy results in a measurable change in the combined sacrum-coccyx shape by comparing midsagittal angles and curvature indices between nulliparous (have never given birth), gravid (pregnant), and parous (have given birth) women. We hypothesized that these groups would differ significantly, consistent with remodeling and coccyx movement that would facilitate vaginal delivery.
2. Methods
This retrospective study was approved by the Institutional Review Board at the University of Pittsburgh and Northshore University HealthSystem. Images of 62 female patients between the ages of 20 and 49 that had a magnetic resonance imaging (MRI) pelvic scan with or without contrast for medical indications (such as abdominal/pelvic pain, appendicitis, abnormal placentation, or fetus anomalies) at Magee-Women’s Hospital or Northshore University HealthSystem between 2005 and 2018 were included in this study. Exclusion criteria were history of pelvic surgery (not including cesarean delivery), pelvic masses, and incomplete scans or birth history. Nongravid patients were imaged in the supine position while gravid patients were imaged in a lateral decubitus position. Patients were sorted into groups based on parity (number of births) and whether they were gravid (pregnant) resulting in 20 nulliparous (had never given birth), 16 gravid and vaginally nulliparous (pregnant and had never given birth vaginally), and 26 parous (had given birth at least once but were not currently pregnant) patients. Using HOROS v. 3.3.5 (Nimble Co LLC, Annapolis, MD USA) the midsagittal slice was identified, and 12 different sacrum and coccyx angle and curvature measurements were made using
A) B)
definitions from previous literature [7]. All of these measures are either a count (the number of coccygeal vertebrae), length (coccygeal straight and curved length), or curvature index (ratio of straight to curved length). A curvature index is the ratio between the straight and curved length multiplied by 100, where the straight length is a straight line through two predetermine anatomical coordinates and the curved length is the average of the anterior and posterior edges of the shape. A structure with a curvature index value of 100 is perfectly straight while those with lower values are more curved. One of the measures that was defined in the previous literature was the sacral angle, but this measure was excluded here as many scans did not include necessary bony landmarks [7]. Three measures of interest are illustrated and defined by Figure 1 where the rest are defined as follows: Coccygeal curved and straight lengths measured from the middle of the upper border of the first coccyx vertebrae (Co1) to the coccygeal tip; Sacral curved and straight lengths measured from the middle of the upper border of S1 to the middle of the of the inferior border of S5; sacrococcygeal curved and straight lengths measured from S1 to the tip of the coccyx; Sacrococcygeal angle is the included angle between the middle of the superior portion of S1, the middle of the superior portion of the Co1, and the tip of the coccyx; Coccygeal angle is the included angle between the line drawn through the superior and inferior portions of Co1 and the line drawn through the superior and inferior of the most inferior coccygeal vertebrae [7].This study defined the sacrum as the 5 vertebrae below the lumbar spine. The sacrum-coccyx border is easily identifiable by the sharp change in orientation of the individual vertebrae, where the entire sacrum-coccyx shape is visible (Figure 1). Everything inferior to the 5 sacral vertebrae was defined as the coccyx, resulting in 3, 4, or 5 coccygeal vertebrae. The number of coccygeal vertebrae was included in the following statistical analysis and the difference between groups was found to be statistically insignificant.
C)
Figure 1: A) Shows the outline of the sacrococcygeal curvature index. The curvature index is the ratio between the straight length (yellow) to the average between the anterior and posterior curved lengths (pink and cyan respectively). B) Shows the sacrococcygeal curvature index the colors and the calculation are the same as in figure 1A. C) Shows the sacrococcygeal angle which is the included angle between the sacral and coccygeal straight lengths.
Statistical analyses were conducted in SPSS v.25 (IBM Corp. IBM SPSS Statistics, Armonk, NY) and consisted of a OneWay Independent MANCOVA followed by univariate ANOVAs and Benjamini-Hochberg (BH) corrections post hoc [8]. The covariates were age and parity. In a BH correction, a critical value is calculated, and if the p-value is smaller the measure is considered significant. Those variables are then considered in a univariate analysis. Measures with significant differences between groups (p<0.05) were followed-up with additional multiple comparisons. Homogeneity of variances were tested, and independent samples were assumed.
3. Results
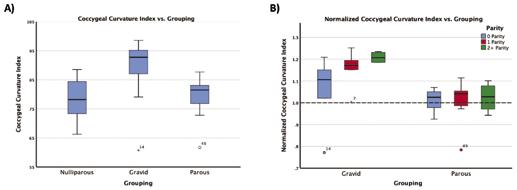
Overall, the sacrococcygeal measures between groups (nulliparous, gravid, and parous) were significant at the multivariate level (p<0.001). After BH corrections, three of the measures had significant univariate results: the coccygeal curvature index (p<0.001), sacrococcygeal curvature index (p<0.001), and sacrococcygeal angle (p=0.010). Table 1 shows the measures (average and standard deviation), the univariate p-values, and the BH critical values for all measures. The data shows that the sacrum-coccyx shape becomes straighter and the sacrum and coccyx more aligned during pregnancy. For all significant measures, only the nulliparous and gravid groups differed significantly (Figure 2a).
When further separating those groups into subgroups based on parity, we can isolate the effect of pregnancy from that of vaginal delivery (Figure 2b). Because our gravid patients were vaginally nulliparous, this refers to non-vaginal delivery methods (i.e. cesarean delivery). However, the parous group was separated by vaginal parity. Qualitatively, we see that these subgroups differ more within the gravid group.
Measure
Coccygeal Curvature Index Sacrococcygeal Curvature Index Sacrococcygeal Angle Coccygeal Straight Length Sacrococcygeal Straight Length Sacral Curvature Index Sacral Straight Length Sacral Curved Length Coccygeal Angle Coccygeal Curved Length Coccygeal Vertebrae Sacrococcygeal Curved Length Nulliparous (Mean ± std) 78.7 ± 6.6 73.3 ± 5.8 92.8° ± 10.9° 3.2 cm ± 0.5 cm 11.4 cm ± 1.2 cm 90.7 ± 6.6 10.8 cm ± 0.8 cm 12.0 cm ± 0.8 cm 126.3° ± 17.2° 4.1 cm ± 0.6 cm 3.5 ± 0.7 15.6 cm ± 1.0 cm Gravid (Mean ± std) 89.2 ± 10.0 79.2 ± 3.7 109.3° ± 9.4° 3.7 cm ± 0.8 cm 12.3 cm ± 0.9 cm 89.2 ± 3.6 10.5 cm ± 1.0 cm 11.8 cm ± 0.9 cm 132.4° ± 21.9° 4.2 cm ± 0.8 cm 3.4 ± 0.5 15.5 cm ± 1.4 cm
Table 1: Table of all sacrococcygeal measures with significant differences bolded Parous (Mean ± std) 80.0 ± 5.5 77.6 ± 5.4 101.9° ± 11.0° 3.2 cm + 0.5 cm 11.9 cm +1.2 cm 92.6 ± 4.6 10.7 cm ± 0.9 cm 11.7 cm + 0.8 cm 133.9° ± 14.5° 4.1 cm ± 0.6 cm 3.5 ± 0.6 15.4 cm ± 1.1 cm ANOVA p-value < 0.001 < 0.001 0.010 0.027 0.123 0.140 0.550 0.662 0.749 0.765 0.777 0.821 B-H CV 0.004 0.008 0.013 0.017 0.021 0.025 0.029 0.033 0.038 0.042 0.046 0.050
Figure 2: A) boxplot of the coccygeal curvature index values with respect to their grouping (nulliparous, gravid, parous) with significant p-values shown B) shows the coccygeal curvature index normalized to the nulliparous average (shown by the dotted line at y = 1) separated by parity. For the gravid (which are vaginally nulliparous) the parity is non-vaginal delivery while the parous group is separated by number of vaginal deliveries.
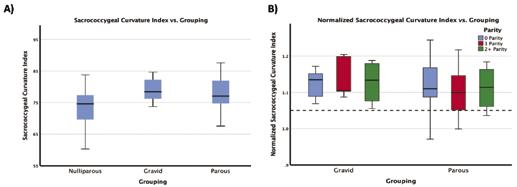
Figure 3: A) boxplot of the sacrococcygeal curvature index values with respect to their grouping (nulliparous, gravid, parous) with significant p-values shown B) shows the coccygeal curvature index normalized to the nulliparous average (shown by the dotted line at y = 1) separated by parity. For the gravid (which are vaginally nulliparous) the parity is non-vaginal delivery while the parous group is separated by number of vaginal deliveries.
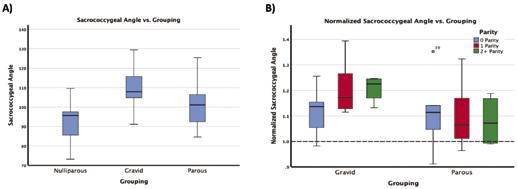
Figure 4: A) boxplot of the sacrococcygeal angle values with respect to their grouping (nulliparous, gravid, parous) with significant p-values shown B) shows the coccygeal curvature index normalized to the nulliparous average (shown by the dotted line at y = 1) separated by parity. For the gravid (which are vaginally nulliparous) the parity is non-vaginal delivery while the parous group is separated by number of vaginal deliveries.
The second significant measure was the sacrococcygeal curvature index (Figure 3). The figure shows that gravid group trends to have a straighter sacrum and coccyx (trending towards 100 on the y-axis). The values follow a similar trend to that of the coccygeal curvature index, as the nulliparous and gravid groups differed significantly while the parous group fell in between, differing significantly from neither.
The third significant measure was the sacrococcygeal angle (Figure 4). Again, only the nulliparous and gravid groups differed significantly. A similar trend is followed as well when the groups are split by parity as subsequent non-vaginal deliveries in the gravid group resulted in larger shape changes.
4. Discussion
Overall these results support the hypothesis that pregnancy and childbirth result in significant change to the combined maternal sacrum-coccyx shape. Specifically, the coccyx moves posteriorly with respect to the sacrum during pregnancy as the coccygeal and sacrococcygeal curvature indices of gravid patients are closer to 100. This is reinforced by the fact that the sacral measurements did not differ significantly across any of the groups. This suggests that pregnancy and delivery would influence the coccyx more than the sacrum.
These shape changes appear to meaningfully increase with each subsequent pregnancy. Figure 2b shows the coccyx becomes straighter (curvature index closer to 100) with subsequent pregnancies following Cesarean deliveries. However, the parous group does not differ from the nulliparous average, even after multiple deliveries. A curvature index closer to 100 shows that as the fetus grows the in the mother the sacrum/coccyx shape straightens out to become more vertical within the mother. Previous work has shown a similar phenomenon with cellular memory that results in many changes within the mother that allow for easier deliveries and postpartum recovery [9]. Another supports our findings that there is recovery in women after delivery, as at one year postpartum the mothers’ bodies had recovered significantly [10].
In future research, longitudinal studies should focus on the effect of multiple pregnancies and mode of delivery to further explain the differences seen here. As the major limitation of this study is that the women are not the same across all groups. A future longitudinal study would be able to confirm the findings of this study by following the same patient throughout pregnancy and after delivery. This study only looked at midsagittal shape differences but provides motivation for a 3D analysis to investigate shape variations in the entire bony pelvis.
5. Conclusions
This study found that the coccyx is the main cause of variation in the combined maternal sacrum-coccyx shape as the coccyx moves posteriorly with respect to the sacrum during pregnancy. For some women, these changes persist after delivery, while others return to a more nulliparous shape. Continuation of this study may lead to an increase in understanding the ways that women are injured during delivery.
6. Acknowledgements
I would like to thank Dr. Abramowitch and Megan Routzong for all of their help with research this past summer. I would also like to acknowledge the Swanson School of Engineering undergraduate research grant and NSF GRFP Grant #1747452 for supporting this research.
7. References
[1] P. Soma-Pillay, P. Soma-Pillay, C. Nelson-Piercy, H. Tolppanen, A. Mebazaa, and A. Mebazaa, “Physiological changes in pregnancy : review articles,” Cardiovasc. J. Afr., 2016, doi: 10.5830/cvja-2016-02.
[2] M. R. Routzong, P. A. Moalli, S. Maiti, R. De Vita, and S. D. Abramowitch, “Novel simulations to determine the impact of superficial perineal structures on vaginal delivery,” Interface Focus, 2019, doi: 10.1098/rsfs.2019.0011.
[3] S. D. Liddle and V. Pennick, “Interventions for preventing and treating low-back and pelvic pain during pregnancy,” Cochrane Database of Systematic Reviews. 2015, doi: 10.1002/14651858. CD001139.pub4.
[4] T. Sipko, D. Grygier, K. Barczyk, and G. Eliasz, “The Occurrence of Strain Symptoms in the Lumbosacral Region and Pelvis During Pregnancy and After Childbirth,” J. Manipulative Physiol. Ther., 2010, doi: 10.1016/j.jmpt.2010.05.006. [5] J. Borg-Stein and S. A. Dugan, “Musculoskeletal Disorders of Pregnancy, Delivery and Postpartum,” Physical Medicine and Rehabilitation Clinics of North America. 2007, doi: 10.1016/j. pmr.2007.05.005.
[6] M. Alperin, T. Kaddis, R. Pichika, M. C. Esparza, and R. L. Lieber, “Pregnancy-induced adaptations in intramuscular extracellular matrix of rat pelvic floor muscles,” Am. J. Obstet. Gynecol., 2016, doi: 10.1016/j.ajog.2016.02.018. [7] J. T. K. Woon, V. Perumal, J. Y. Maigne, and M. D. Stringer, “CT morphology and morphometry of the normal adult coccyx,” Eur. Spine J., 2013, doi: 10.1007/s00586-012-2595-2. [8] Y. Benjamini and Y. Hochberg, “Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing,” J. R. Stat. Soc. Ser. B, 1995, doi: 10.1111/j.2517- 6161.1995.tb02031.x.
[9] D. Goldman-Wohl, M. Gamliel, O. Mandelboim, and S. Yagel, “Learning from experience: cellular and molecular bases for improved outcome in subsequent pregnancies,” American Journal of Obstetrics and Gynecology. 2019, doi: 10.1016/j. ajog.2019.02.037.
[10] C. Reimers, J. Stær-Jensen, F. Siafarikas, J. SaltyteBenth, K. Bø, and M. Ellström Engh, “Change in pelvic organ support during pregnancy and the first year postpartum: A longitudinal study,” BJOG An Int. J. Obstet. Gynaecol., 2016, doi: 10.1111/1471-0528.13432.










