
14 minute read
u Representations of population activity during sensorimotor transformation for visually guided eye movements
Representations of population activity during sensorimotor transformation for visually guided eye movements*
Eve C. Ayara,c,d, Michelle R. Heusserb,d , Neeraj J. Gandhia,b,c,d
aDepartment of Neuroscience, bDepartment of Bioengineering, c Center for Neuroscience (CNUP), d Center for Neural Basis of Cognition (CNBC)
Eve Ayar Eve Ayar is a junior Neuroscience student. She is interested in investigating patterns of population activity during sensorimotor integration. After graduation, she plans to pursue a Ph.D. in Neural Computation.
Michelle R. Heusser Michelle Heusser is a Ph.D. candidate in Bioengineering at the University of Pittsburgh. Her research in the Gandhi lab focuses on the time course of neural population activity in the superior colliculus and its relationship to motor behavior.
Neeraj (Raj) Gandhi, Ph.D. Neeraj (Raj) Gandhi, Ph.D. is Professor and Graduate Program Director in the Department of Bioengineering. He also developed the Professional Masters program focused on neural engineering. His research focuses on understanding neural communication during sensation, action, and cognition.
Significance Statement
Sensorimotor transformation is a process that humans perform over 100,000 times a day—for example, when we look at or reach for objects of interest. Many areas of the brain register a sensory stimulus and convert the stimulus-related information into an appropriate motor output. Deficits in sensory and/or motor processes are implicated in a number of neurological disorders such as Parkinson’s Disease [1]. We are interested in how the context of visual behavioral tasks impacts patterns of population activity during sensorimotor transformation.
Category: Computational Research
Keywords: saccade, dimensionality reduction,
eye movement, sensorimotor
Abbreviations: superior colliculus (SC), peristimulus
time histogram (PSTH)
*Reviewers’ Choice
Abstract
For visually guided eye movements known as saccades, neurons in the superior colliculus in the midbrain emit a volley of action potentials to register a visual (sensory) stimulus, and they also discharge another high frequency burst of spikes to move the line of sight. We investigated the representations of sensory- and motor-related population activity during two paradigms, the delayed saccade and gap tasks. The two tasks differ in instructing the time to initiate the eye movement, permitting different temporal evolutions of the sensorimotor transformation. Dimensionality reduction methods were used to visualize the pattern of activity of recorded neurons and determine if the population responses in both tasks exhibit similar visual and motor patterns despite differences in event timing and cognitive context. Preliminary analyses suggest that the visual patterns explored during the delay and gap tasks largely overlap, while patterns of motor activity present differences, leading us to believe that downstream structures may differentiate signal processing between behavioral tasks.
1. Introduction
Consider a monkey in a tree searching for a banana. If he sees something yellow appear in his visual field, he will look at it. Simply put, this is the process of sensorimotor transformation: the brain registers a sensory input (e.g., banana) and then converts it to a motor output (e.g., the act of looking at or reaching for the banana). The superior colliculus (SC) is a structure in the brain that is integral in this process because it contains both visual and motor related signals that rapidly redirect the visual axis. We want to understand how sensorimotor transformation occurs in different tasks. If the context of a behavioral task matters, we expect to see differences in SC neural activity patterns because the signals involved in sensorimotor transformation will be processed differently.
To characterize different representations of population activity, neural activity patterns were analyzed across two conditions: the delayed saccade task and gap task (Figure 1). The delayed saccade task requires the animal to volitionally withhold movement generation until a later point in time. This condition separates the visual and motor bursts in time. The gap saccade task, in contrast, requires the animal to react immediately. Thus, the movement can happen as soon as visual signals are processed. In this condition, the visual and motor neural bursts can temporally overlap and may even merge into a single burst. For both tasks, we care about activity at two key time points in a trial, 1) estimated visual burst time and 2) saccade onset, indicated by the lines in Figure 1. These key points occur in both tasks but have different timelines because one task has an imposed delay, and the other does not.
In this project, we focus on the similarities and differences between visual and motor bursts under the two conditions in a low-dimensional state space, with the goal of determining whether there is a different pattern of population activity relayed to downstream areas.
We hypothesize that the gap task’s population response will likely have a different pattern of motor activity than the delayed saccade task. If this hypothesis is supported, this may mean that the context of a behavior matters – the pattern of SC neural activity underlying a behavior will change due to the differing conditions of the task. However, it is possible that context does not matter, and patterns of activity will be similar. In this case, downstream structures may not differentiate how neural signals are processed depending on the context of the behavior.
Figure 1. Task timeline schematics and key for a typical the delayed saccade (left) and gap task (right) trial.
2. Methods
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and followed the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals. A rhesus monkey (Macaca mulatta) underwent surgery to implant a recording chamber and allow access to the SC. On each recording session, a 16-channel linear microelectrode array (AlphaOmega Inc.) was inserted orthogonal to the SC surface of the monkey to record a small population of neurons.
Every trial is initiated by presentation of a fixation point usually at the center of the screen, and the animal is required to direct its line of sight on this stimulus. In the delayed saccade task (Figure 1A), a single target, as described earlier, is presented while the monkey continues to fixate on the central stimulus. Then the fixation point is turned off, acting as the cue to make a movement to the target. This time in between the target appearance and the go cue is defined as the “delay” period which can be anywhere between 600-920 ms for each trial. The length was varied in order to keep the animal guessing when to make an eye movement, which served to minimize anticipatory responses. In the gap task (Figure 1B), the fixation point is turned off and a gap in time occurs before a single target is presented on the screen, acting as the cue to make a movement to the target. In both tasks, 16 channels of activity were recorded. We’ll refer to this as our neural population, as the channels were inspected for task-related activity consistent with the characteristic activity profile of neurons located in the SC. The target was presented at a location for which the majority of SC neurons being recorded emit many spikes.
In both tasks, the key points in each trial are visual burst time and saccade onset. For the delayed saccade task, the visual burst peak time (estimated to be 160 ms across all trials, determined visually using PSTHs) is represented with blue and saccade onset with red (Figure 2A). For the gap task, the visual burst time (estimated to be 140 ms across all trials) is represented with cyan and saccade onset (determined using each trial’s measured reaction time) with pink (Figure 2B). These colors are used throughout the rest of our analyses comparing the neural activity underlying these key external events.

Figure 2. High-dimensional neural activity during the delayed saccade and gap tasks. (A) Peristimulus time histogram (PSTH) of high dimensional activity during the delayed saccade divided into two plots: time in trial aligned to target onset and time aligned to saccade onset. The visual burst time is represented by the blue line (approximated to be 160 ms after target onset) and the motor burst is represented by the red line (at saccade onset, 0 ms). (B) PSTH of high dimensional activity during the gap task. The visual burst is represented by the cyan line (approximated to be 140 ms after target onset) and the motor burst is represented by the pink rectangle. (A, B) There are 16 channels, and all appear to have task-related activity.
Each trial was stored for analysis in MATLAB. Two data sessions were used in these analyses (02/08/17, 03/18/17). Both sessions showed task-related neural activity in all 16 channels. Dimensionality reduction was used to summarize the population activity of many neurons. This allows us to better visualize neural activity and compare across tasks. Specifically, Gaussian Process Factor Analysis (GPFA) was used on 16-channel spike trains aligned to target onset with a 20 ms smoothing factor [2]. Operating on a high-dimensional dataset, GPFA extracts a reduced number of factors that account for a large proportion of the variance and, additionally, it smooths these factors in time. Temporal smoothing while simultaneously performing dimensionality reduction provides a more intuitive visual representation of how activity evolves over time and why activity for closer key points of a given trial are more similar that activity for those further apart in a given trial. We applied the GPFA algorithm to delayed saccade task trials only; gap task trials were then projected into the same low-dimensional space to allow for a fair comparison of neural activity by ensuring that the weights attributed to each neuron for both tasks are the same [3].
Once the activity was summarized in a low-dimensional space, the Euclidian distances between the means of similar activity for each task were calculated. The raw data was then normalized by the Euclidean distance between the means of the visual and motor activity for the delayed saccade task. 95% confidence ellipsoids were plotted along with these means in a low-dimensional space. Histograms were created for each latent factor to analyze the separation of activity at the key events across tasks for a given factor.
3. Results
So far, analyses have been performed on two data sessions (panel A/C and panel B/D, respectively, in Figure 3). As expected, based on preliminary findings in our lab, the visual subspace is separable from the motor subspace within each task condition, indicative of a unique neural population activity pattern during the respective sensory and motor behavioral epochs. Interestingly, there is greater overlap for the visual activity for the gap and delayed saccade tasks (cyan asterisks and blue dots, respectively, in Figure 3A) while the motor activity has less overlap across the tasks (pink asterisks and red dots, respectively, in Figure 3A). In session 2, the visual activity has some overlap whereas comparatively, the motor activity is essentially non-overlapping (Figure 3B). In both sessions, the visual activity overlap is greater than the motor.
Figure 3. Comparison of the population activity at key events for the gap and delayed saccade tasks. After dimensionality reduction, three latent dimensions were retained. (A, B) Dots and asterisks represent delayed saccade and gap latent activity, respectively, and each point is 20 ms summary of population activity. (C, D) 95% confidence ellipsoids for visual and motor latent activity along with their respective means. Blue/cyan represent visual and red/pink represent motor, which are the key events that are being compared.
To allow for better visualization, 95% confidence ellipsoids were plotted for the visual and motor activity in both the gap and delayed saccade tasks along with their respective means (Figures 3C and 3D). Again, there is greater overlap in both sessions across the two tasks for visual activity (blue ellipsoids) than for motor activity (red ellipsoids, Figures 3C and 3D).
To further analyze the separation of visual and motor activity across the two tasks, the Euclidean distances between the means of each cluster (visual vs. visual and motor vs. motor) were calculated. These raw distances were then normalized using the Euclidean distance between the mean of visual and motor activity in the delayed saccade task (i.e., the task condition with the greatest separation). Table 1 displays these distances. The values agree with our results in Figure 3, in which the distance between the motor clusters (0.6453) is greater than the distance between visual clusters (0.5561).
Table 1: Euclidean Distances Between Means of Activity for Delayed Saccade and Gap Tasks
Raw Normalized
Visual Burst 2.7004
Saccade Onset 3.1335 0.5561
0.6453
Table 1. Euclidean distances measured between the means of visual and motor points to compare separation across tasks. The raw measurements are normalized using the Euclidean distance between the means of visual and motor activity during the delayed saccade task (4.8560).
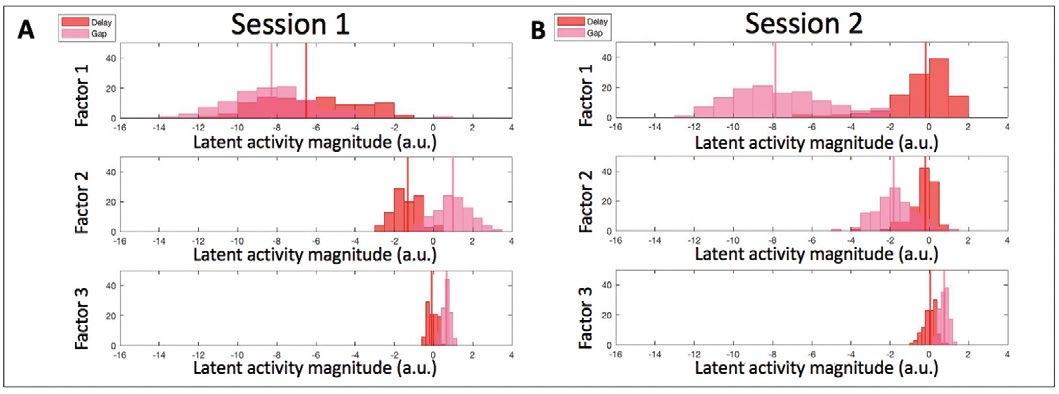
Diving deeper into the data, we analyzed the separation of activity across both tasks in each individual latent dimension. In plotting histograms for both tasks for either visual (Figure 4) or motor (Figure 5) activity we find via visual inspection that there are roughly normal distributions of latent magnitude values along each factor. Given that these data are preliminary, we did not perform a statistical test to determine normality. There was separation for both visual and motor epochs in the first factor, with a large amount of separation for motor, as expected. In session 1 (Figure 5A), there was also greater separation for motor activity in the second factor in comparison to visual activity for that session. This is true, although less so for the second session, and a similar trend can even be seen in the third factor for both sessions.

Figure 4. Comparison of the latent activity magnitude (a.u.) along each latent dimension retained after GPFA for the visual activity during the gap and delayed saccade tasks. The vertical lines represent the medians of visual activity. Same color convention as Figure 3.
Figure 5. Comparison of the latent activity magnitude (a.u.) along each latent dimension retained after GPFA for the motor activity during the gap and delayed saccade tasks. The vertical lines represent the medians of motor activity. Same color convention as Figure 3.
4. Discussion
In the delayed saccade task, the target appears before the go cue is presented so sensorimotor transformation has already started. The delay period essentially increases the amount of time neurons have to evolve their activity. In the gap task, in contrast, there is no delay period; thus, the motor command can initiate as soon as visual signals are processed. Given the inherent complexities of the neural dynamics, the short time period of sensorimotor transformation may limit a thorough evolution of population activity in this task. We observed less separation of visual activity between the two tasks, an expected result because the timing of the visual burst is similar in each. In the gap task, the motor burst often overlapped or merged with the visual burst. The merged bursts reflect why the low-dimensional representation of data show similar patterns across the visual and motor activity for the gap task and less so for the delayed saccade task. Each individual neuron in the gap task does not have much time to evolve its activity. Thus, when looking across the whole population of SC neurons, we can infer that the population as a whole does not evolve its activity in such a short period of time. Other studies have looked at the specific patterns across individual latent dimensions and have deduced that each factor may represent a different neural or behavioral property [4]. In our study, we find that there is greatest separation in the first factor; however, the second and third factors also show separation. With GPFA, there is not a specific mapping between the factors and what properly they represent. In future work, we plan to use other algorithms such as demixed principal component analysis (dPCA) to explore what each of these dimensions may represent, which may provide insight to the differing patterns of neural activity that underly different behaviors [4].
5. Conclusions
Our current results imply the exact distribution of population activity during sensorimotor transformation differs across task conditions. This supports the idea that downstream structures may differentiate how signals are processed in response to different behavioral conditions. For instance, these signals ascend through the thalamus to the frontal cortex, which could potentially discern the different cognitive demands of the two tasks. Thus, the context of a behavior matters when considering how sensorimotor transformation occurs. The same signals also descend to the saccade generator region in the brain stem. The observation that the motor subspaces associated with the two tasks are not identical, albeit partially overlapping, indicates that the actual motor subspace may be larger than that characterized from an individual paradigm. These notions are ripe for future investigations.
6. Acknowledgements
This project was supported by NIH Grants EY024831 and EY022854, and NIH MH 5R90DA023426-15 Interdisciplinary Training in Computational Neuroscience. I would like to acknowledge the members of Dr. Gandhi’s Cognition and Sensorimotor Integration Lab.
7. References
[1] Pretegiani, E., & Optican, L. M. (2017). “Eye Movements in Parkinson’s Disease and Inherited Parkinsonian Syndromes,” in Frontiers in neurology, 8, 592, doi. org/10.3389/fneur.2017.00592 [2] Yu, B. M., Cunningham, J. P., Santhanam, G., Ryu, S. I., Shenoy, K. V., & Sahani, M. (2009). “Gaussian-process factor analysis for low-dimensional single-trial analysis of neural population activity,” in Journal of neurophysiology, 102(1), 614–635, doi.org/10.1152/jn.90941.2008 [3] Cowley, B. R., Kaufman, M. T., Butler, Z. S., Churchland, M. M., Ryu, S. I., Shenoy, K. V., & Yu, B. M. (2013). “DataHigh: graphical user interface for visualizing and interacting with high-dimensional neural activity,” Journal of neural engineering, 10(6), 066012, doi. org/10.1088/1741-2560/10/6/066012 [4] Kaufman, M. T., Churchland, M. M., Ryu, S. I., & Shenoy, K. V. (2014). “Cortical activity in the null space: permitting preparation without movement,” in Nature neuroscience, 17(3), 440–448, doi.org/10.1038/nn.3643










