10 minute read
Immune alteration using human mesenchymal stem cells in schizophrenia: a review
Xiaoke Jiang
Schizophrenia is a serious mental disorder characterized by positive, negative and cognitive symptoms, the word “Schizophrenia” is proposed almost a century ago, yet we do not know the pathology behind it. There are many lines of evidence pointing out the correlation between microglial activation and neuroinflammation in schizophrenia proposing the control of microglial activation potentially could be a therapeutic direction in schizophrenia treatment. In this review, we assess the potential use of human mesenchymal stem cells with its immunosuppression and immunomodulatory property to decrease schizophrenia-relevant behavioral abnormality targeting microglial activation. This review answers two questions, “Can cytokine level be a diagnostics marker in schizophrenia” and “Can human mesenchymal stem cells be a therapeutic drug for schizophrenia” Using the answers to these two questions, criticalanalysisandfutureprospectofclinicalinterpretation isproposed.
Advertisement
Schizophrenia is a complex, serious mental disorder affecting approximately 1% of the population, it is characterized by positive symptoms such as delusions, hallucinations and paranoia, negative symptoms such as social withdrawal and emotional ery and the brain of schizophrenic patients, which is normalized
withdrawal, and cognitive symptoms such as learning and attention disorders. Abnormalities in dopaminergic receptors (e.g., D2 receptor) have established the current understanding of pathophysiology of schizophrenia, although antipsychotic (APDs) medications (both first- and second-generation) aiming dopamine transmission by blocking D2 receptor could alleviate positive symptoms of schizophrenia, frequent occurring negative and cognitive symptoms remain untackled by current APDs. Moreover, these medications have also been associated with side effects including rigidity, tremor, weight gain, metabolic dysregulation and sedation, suggesting an increasing need for novel of macrophage-derived cytokines TNF-α, IL-6and IL-1β in periph
therapeutic approaches and targets for schizophrenia. after APDs treatment. The study considered APDs and clinical status to eliminate potential confounding and the overall conclusion is consistent with the immune-cytokine hypotheses of schizophrenia
Figure 1 Immunohistochemical staining of microglial cell (A) Immunohistochemical staining from frontal cortex of control (C) Immunohistochemical staining from frontal cortex of schizophrenia patients
Adapted fromBayer, Thomas A, Rolf Buslei, Laszlo Havas, and Peter Falkai. “Evidence for Activation of Microglia in Patients with Psychiatric Illnesses.” Neuroscience Letters 271, no. 2 (August 20, 1999): 126–28. https:// doi.org/10.1016/S0304-3940(99)00545-5.
The abnormality of cytokine level have been proposed to be associated with schizophrenia in many studies, although that level of cytokine level often correlate with the severity of clinical symptoms, elevation of puerperal level of IL-1β, IL-6, and TNF-α are concise in schizophrenia patients contrast to control suggesting immune-inflammatory mechanism related to these proinflammatory cytokine maybe involved in schizophrenia pathology.
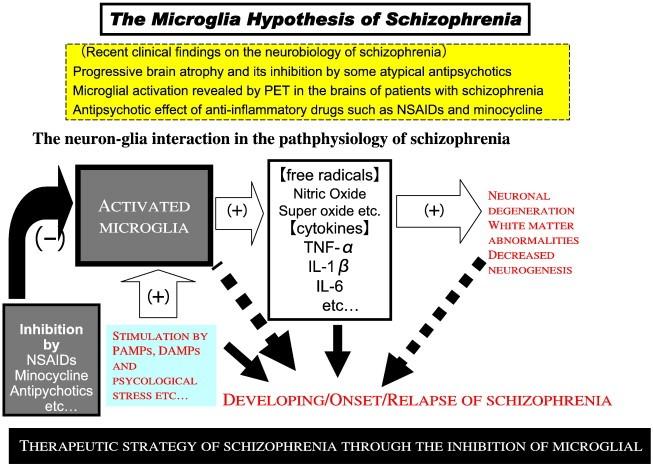
However, although all studies above are informative, they are also innately limited, as the pathology behind schizophrenia remains unknown. This review focuses on linking neuroinflammation with microglial activation in schizophrenia. We provide a review on experiments done by You et al. on stem cell therapy on schizophrenia suggested the potential use of immunomodulatory medication/therapy targeting microglial activation as future treatment for schizophrenia, followed by critical analysis and future prospect. The microglia hypothesis of schizophrenia proposing inhibition of free radical cytokines including TNF-α, IL-6 and IL-1β to treat developing/onset/relapse of schizophrenia was also proposed in a review article (Fig.2), the article showed extensive knowledge in schizophrenia related neuroinflammation focus on the role on microglial activation and suggested controlling anti-inflammatory cytokine as well as immunomodulatory drug as future treatment for schizophrenia .
Results Microglial activation and cytokine
Psychopharmacology and Biological Psychiatry, Special Issue: Inflammato
More recent studies have suggested activated microglial cells associated with neuroinflammation maybe involved in neuropsychiatric disorders like schizophrenia. The idea of microglia activation in brains of patients with severe psychiatric illnesses like schizophrenia was first proposed two decades ago by Thomas A cess for schizophrenia, post-mortem study was conducted using immunohistochemical staining and HLA-DR antigen as an indicator for microglial reactivity. Out of 14 patients with schizophrenia, three exhibited activated microglial cells and all positive cases were late onset schizophrenia, suggesting microglial activation as a key factor in evidence of pathologic changes in the brains of severe psychiatric illnesses patients including some subgroups of schizophrenia patients (Fig.1). A meta-analysis of cytokine alteration found significant increase in circulating level
Fig.2 The microglial hypothesis of schizophrenia Adapted fromMonji et al. “Neuroinflammation in Schizophrenia Especially Focused on the Role of Microglia.” Progress in NeuroBayer, who brought the first insight into pathophysiological pro
ry Pathways as new drug targets in Schizophrenia, 42 (April 5, 2013): 115–
Human mesenchymal stem cells
The use of human mesenchymal stem cells (MSCs) were reported known for possessing numbers of therapeutic effect with its immunomodulatory properties. MSCs are multipotent stem cells that can derived into varieties of cell types such as neuronal cells, with its immunosuppression and immunomodulatory properties, MSCs can regulate regulatory T cells, supress T cell proliferation, cytokine secretion, cytotoxicity and promotes cellmediated immune response (details shown in Fig.3) . MSCs have

In conclusion, although the current understanding of the pathology of schizophrenia still remains unknown and to our current knowledge, there is no single-target drug, however neuroinflammation and microglial activation might be a key pathologic marker for diagnostics in schizophrenia, experiments performed above suggested stem cell therapy using MSCs maybe effective for schizophrenia patients with elevated TNF-α, controlling inflammatory cytokine signaling maybe a new therapeutic approach for schizophrenia.
Fig.3(Previous page) MSCs immunomodulatory effect on immune cell. Adapted from Gao, F., Chiu, S., Motan, D. et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death however heterogeneous schizophrenic disorder group cannot
Dis 7, e2062 (2016). https://doi.org/10.1038/cddis.2015.327
Critical Analysis
Animal models
cell-therapy, etc. The article we reviewed proposed a potent therapeutic alteration using human umbilical cord derived MSCs for treating neuroinflammation in schizophrenia. MSCs has been reported to possess immunomodulatory properties in schizophrenia in a meta-analysis for its efficiency and safety, results were collected double-blinded, internationally to eliminate potential bias. Based on this conclusion, the first experiments using MSCs for reducing clinical symptoms of schizophrenia using mice model was performed and the results were Due to the multi-factorial property of schizophrenia, there is no “perfect” animal model as the genetic differences often lead to limited interpretation from animals to humans, the author used AMP mice to induce schizophrenia-relevant behaviour,
interpreted. be represented. Several studies have shown elevated peripheral IL-6 circulation in schizophrenia patients (Table.1),

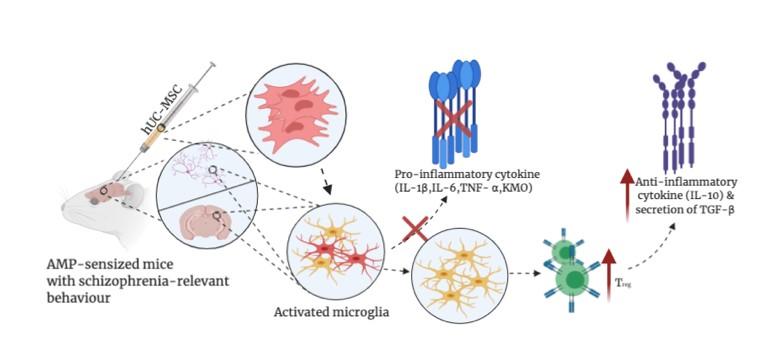
The immunomodulatory effect of MSCs was associated by the introduction of regulatory T cells with the production of antiinflammatory cytokine IL-10 and prohibit the sustained neuroinflammation cause by the elevation of mRNA expression of TNF-α, IL-1β, and KMO in amphetamine-sensitized mice (AMP mice) with schizophrenia-relevant behaviour (Fig.4). Vitro study concluded that the neuroinflammation and microglial activation maybe correlated with elevation is circulating TNF-α instead of by the direct effect of amphetamine which was inhibited by MSCs. Behavioural test performed after MSCs injection in AMP mice showed some degree of decrease in behavioural abnormality proposing a possible therapeutic option in schizophrenic subgroup with elevated TNF-α.
Table.1 Summary of cytokine level in schizophrenia patients based on metastudy. Adapted from Momtazmanesh, Sara, Ameneh Zare-Shahabadi, and Nima Rezaei. “Cytokine Alterations in Schizophrenia: An Updated Review.” Frontiers which is contradicting to the non-significant result of IL-6 in the experiment You et al. conducted (Fig.5). Moreover, the contention of using prepulse inhibition (PPI) in detecting schizophrenia is well
Adapted from You et al. “Human Umbilical Cord-Derived Mesenchymal Stem Cells Alleviate Schizophrenia-Relevant Behaviors in AmphetamineSensitized Mice by Inhibiting Neuroinflammation.” Translational Psychiatry 10, no. 1 (December2020): 123. https://doi.org/10.1038/s41398- 020-0802-1. established and PPI deficits are identified to be one of the most distinguished characteristics in schizophrenia patients. In contrast to this founding, You et al.’s finding reported to show no PPI deficits in AMP mice compare to control. From the review above, it is apparent that genetically modified rodent model or
Fig.4 Underlying mechanism of MSCs in AMP mice neurodevelopmental rodent model would be more appropriate for studying schizophrenia. The prenatal methylazoxymethanol acetate (MAM) rodent model would be more relevant since it epitomizes developmental disruption bringing out histological, neurophysiological and behavioral deficits more closely resem
bles to schizophrenia, and more comprehensive mechanism behind the pathology of schizophrenia and effect of MSCs on microglial activation can be well studied.
IL-10
Anti-inflammatory cytokine IL-10 have been explored to have an association with schizophrenia. A meta-study has shown single nucleotide polymorphisms (SNPs) of IL-10 significantly correlate with the risk factor for schizophrenia, and level of IL10 is seen to be negatively associated with severity of clinical symptoms of schizophrenia particular in first episode and drug -naive (FEDN) psychosis and first episode psychosis (FEP). Moreover, in a different study performed by Lee et al. on the effect of MSCs on Niemann-Pick type C (NP-C) disease, the effect of MSCs is also reported to be associated with increase of IL-10 circulation to modulate inflammation response. All pointing out increase in IL-10 circulation may have an effect on microglial activation. In vitro study performed by You et al. who hypothesized IL-10 to exert similar effect of MSCs but found no effect of IL-10 infusion rescue schizophrenia-relevant behavior. The author should perform further study to block the effect of IL-10 using anti-IL-10R monoclonal antibody then test the effect of MSCs and to gain deeper understanding behind the mechanisms of MSCs.
Future studies
To resolve these gaps, proper mice model (suggest MAM mice model) for schizophrenia must be used in future studies and to test for the role of IL-10 in modulating neuroinflammation in schizophrenia, MAM mice must be inject with MSCs first followed by IL-10 antibody injection to test for behavioural and vitro studies. Furthermore, the experiment should have longer experimental duration to see whether MSCs could potentially have a long-term effect. If the results show no degree of behavioural rescue and persistent neuroinflammation, it can be interpreted that MSCs without inducing IL-10 itself may not have the direct effect on microglial activation modulation. Vitro study using cultured microglial cell lines can be performed, thus the role of MSCs and IL-10 must be concisely studied. Moreover, it may be beneficial to repurpose other immunomodulatory drug/therapy previously proposed for autoimmune diseases in severe psychosis like schizophrenia. Taken together, additional studies are required before MSCs can be considered an efficient therapeutic strategy for schizophrenia.
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Bayer, Thomas A, Rolf Buslei, Laszlo Havas, and Peter Falkai. “Evidence for Activation of Microglia in Patients with Psychiatric Illnesses.” Neuroscience Letters 271, no. 2 (August 20, 1999): 126–28. https://doi.org/10.1016/S0304-3940(99)
00545-5. Chen, Faye H., and Rocky S. Tuan. “Mesenchymal Stem Cells in Arthritic Diseases.” Arthritis Research & Therapy 10, no. 5 (October 10, 2008): 223. https://doi.org/10.1186/ar2514. 1655 (January 15, 2017): 262–69. https://doi.org/10.1016/j.brainres.2016.08.010.
Donegan, Jennifer J., and Daniel J. Lodge. “Cell-Based Therapies for the Treatment of Schizophrenia.” Brain Research Gao, F., S. M. Chiu, D. a. L. Motan, Z. Zhang, L. Chen, H.-L. Ji, H.-F. Tse, Q.-L. Fu, and Q. Lian. “Mesenchymal Stem Cells and Immunomodulation: Current Status and Future Prospects.” Cell Death & Disease 7, no. 1 (January 2016): e2062–
e2062. https://doi.org/10.1038/cddis.2015.327. Gao, Lei, Zhao Li, Suhua Chang, and Jing Wang. “Association of Interleukin-10 Polymorphisms with Schizophrenia: A Meta -Analysis.” PLOS ONE 9, no. 3 (March 6, 2014): e90407. https://doi.org/10.1371/journal.pone.0090407. Kállai, Veronika, Attila Tóth, Rita Gálosi, László Péczely, Tamás Ollmann, Zoltán Petykó, Kristóf László, et al. “The MAMBrain Research 332 (August 14, 2017): 75–83. https://doi.org/10.1016/j.bbr.2017.05.065.
E17 Schizophrenia Rat Model: Comprehensive Behavioral Analysis of Pre-Pubertal, Pubertal and Adult Rats.” Behavioural Lee, Hyun, Jae-Sung Bae, and Hee Kyung Jin. “Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Improve
Neurological Abnormalities of Niemann-Pick Type C Mouse by Modulation of Neuroinflammatory Condition.” Journal of
Veterinary Medical Science 72, no. 6 (2010): 709–17. https://doi.org/10.1292/jvms.09-0495.
Lodge, Daniel J. “The MAM Rodent Model of Schizophrenia.” Current Protocols in Neuroscience / Editorial Board, Jacqueline N. Crawley ... [et Al.] 0 9 (April 2013): Unit9.43. https://doi.org/10.1002/0471142301.ns0943s63.
Miller, Brian J., Peter Buckley, Wesley Seabolt, Andrew Mellor, and Brian Kirkpatrick. “Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects.” Biological Psychiatry 70, no. 7 (October 1, 2011): 663–71. https://doi.org/10.1016/j.biopsych.2011.04.013.
Momtazmanesh, Sara, Ameneh Zare-Shahabadi, and Nima Rezaei. “Cytokine Alterations in Schizophrenia: An Updated
Review.” Frontiers in Psychiatry 10 (2019). https://doi.org/10.3389/fpsyt.2019.00892. 11. Monji, Akira, Takahiro A. Kato, Yoshito Mizoguchi, Hideki Horikawa, Yoshihiro Seki, Mina Kasai, Yusuke Yamauchi, Shigeto
Yamada, and Shigenobu Kanba. “Neuroinflammation in Schizophrenia Especially Focused on the Role of Microglia.” Progress in Neuro-Psychopharmacology and Biological Psychiatry, Special










