PCR. Pingry Community Research Fall 2022.
Showcasing the next generation of scientific researchers.


Showcasing the next generation of scientific researchers.

Method of Gauss Orbital Determination of Near-Earth Asteroid 2015 HH10 7
UAV and UGV Autonomous Cooperation for Wildfire Hotspot Surveillance 14
Whamy is Involved with Actinmyosin Ring Constriction During Drosophila Cellularization 22
Mycotoxin-Induced Plant Stress and cpUPR in Chlamydomonas reinhardtii 23

The Sonochemical Degradation of PFOA and PFOS 28

Shaming, Stigma, and Povery Surrounding Menstruation in Afghanistan 32
Effects of Facial Expressions on Willigness to Purchase 41
An Exercise-Inducible Metabolite that Suppresses Feeding and Obesity 46
Mechanims of Satellite Glial Cells in Chronic Pain Genesis and Maintenance 47
Glioblastoma Multiforme: A Therapeutic Review 58
Summer Intern at the Coriell Institute for Medical Research 66


Welcome to the Fall 2022 issue of the Pingry Community Research (PCR) Journal. We are delighted to showcase Pingry’s top scientific talent, both in terms of research skills and knowledge of scientific concepts and discoveries.
The PCR journal provides students the opportunity to publish novel research. Through a written medium, students demonstrate their in-depth understanding of complex, collegiate-level scientific topics, and their applications in research at Pingry.
The fall edition of PCR highlights work in two categories: reporter articles, which are written by students on a scientific topic of their choosing, and novel research articles, which communicate the findings of novel research conducted by Pingry students outside of school in a myriad of fields.
Through the PCR journal, we hope to spark intellectual curiosity and promote scientific inquiry amongst the next generation of Pingry researchers.
Read, learn, and inquire. Dive into the wonders of Pingry Research through this issue of PCR: Pingry’s foremost journal of scientific research.
Mirika Jambudi (VI), Editor-in-Chief Evan Xie (V), Head Copy EditorEditor-in-Chief: Mirika Jambudi (VI)
Head Copy Editor: Evan Xie (V)
Copy Editors: Katia Krishtopa (V) Brielle Marques (V) Sophia Odunsi (V) Chelsea Peng (V) Annabelle Shilling (V) Rohan Yadav (IV)

Art Editor: Kain Wang (V)
Faculty Advisor: Mr. Maxwell


1Summer Science Program and Sommers-Bausch Observatory, University of Colorado Boulder, Boulder, CO, 80309, USA
Near-Earth asteroids, a subset of near-Earth objects (NEOs), are characterized by their close approach to the Sun. By definition, an astronomical body is considered an NEO if it comes within 1.3 AU of the Sun at its closest point. If a NEO is believed to make an approach close enough to the Earth to cause significant damage, it is assessed as a potentially hazardous object (PHO). We observed the NEO 2015 HH10 over the course of four nights to determine its orbit. Using observational data and the orbital determination Python program that we developed, we determined its orbital elements and generated an ephemeris of its future trajectory and position. Our methods and procedures are shown to be more precise than the JPL Horizons database.
There is a multitude of different space objects floating around the Solar System, including both natural and artificial space debris. Artificial or manmade debris includes old machinery or dead satellites left behind by human space missions. Natural space debris consists of asteroids and comets which break up into smaller meteoroids. While both asteroids and comets formed early on in the universe about 4.5 billion years ago [1], comets and asteroids differ in composition. Comets are usually made of ice, dust, and other rocky material, populating the Kuiper Belt in the outer edges of the Solar System or even beyond the Solar System in the Oort Cloud [2]. Asteroids are small bodies that orbit the sun, primarily residing in the asteroid belt between Mars and Jupiter. They are usually around 1,000 km or less in diameter and are composed of metals and other rocky materials, such as iron or nickel, as well as elements abundant in the Earth, such as oxygen and silicon [3]. An asteroid must pass within 1.3 AU of the Sun at its closest approach to be considered a near-Earth asteroid [1]. Some Mars-crossing asteroids which lie between the main asteroid belt and the near Earth asteroid population fit within the parameters of NEOs if their perihelion (point in the orbit when the object is closest to the Sun) is less than 1.3 AU. There are several types of near-Earth asteroids, classified by their orbits and locations: Atira asteroids, Atens Asteroids, Apollo asteroids, and Amor asteroids [2]. 2015 HH10 is categorized as an Apollo asteroid which means its orbit crosses Earth’s orbit and it has a semi-major axis greater than Earth’s. Its perihelion is less than the Earth’s aphelion (point in the orbit when the object is farthest from the Sun). Since Apollo asteroids are Earth-crossing, they are interesting targets to study as potentially hazardous objects (PHO). For example, in 2013, the Chelyabinsk meteor (an Apollo asteroid) exploded over rural Russia, injuring an estimated 1,500 people [4]. The Apollo asteroid population forms the majority of currently known PHOs. In order to expand the search for NEOs and predict possi-
ble PHO behavior, the International Astronomical Search Collaboration provides amateur astronomers with data so they can make scientific discoveries, often focusing on month-long Asteroid Search Campaigns in which teams search for asteroids [5]. Search campaigns such as those run by the International Astronomical Search Collaboration encourage international cooperation and the involvement of citizen scientists in real-time astronomical discoveries.
In order to determine the orbit of an asteroid, there are several necessary components, known as the six Keplerian elements: semi-major axis, eccentricity, inclination, longitude of the ascending node, argument of perihelion, and mean anomaly. The semi-major axis measures half of the longer axis in an ellipse, generating the ”long radius” that defines the size of the orbit. The eccentricity is used to describe the elongation of an ellipse; a perfectly circular ellipse has an eccentricity of 0. The inclination is the angle between the asteroid’s plane and the Earth’s plane (ecliptic plane). The longitude of the ascending node is the angle between the Vernal Equinox and the ascending node, the point at which the orbital and ecliptic planes intersect. The argument of perihelion is the angle between the asteroid’s ascending node and perihelion, the point where the asteroid is closest to the Sun. The three angles—inclination, longitude of the ascending node, and argument of perihelion—are known as the Euler angles as they describe the asteroid’s orbit orientation relative to the original reference plane. The mean anomaly is a convenient approximation for the angular position of the object, calculated from specific times of observation, that varies linearly with time. These six orbital elements are the foundation for finding and predicting the trajectory of an asteroid. In addition to the main elements, the time of perihelion (date and time at which an object is closest to the Sun) can be derived. The orbital state vectors (Cartesian vectors of position and velocity) produced by the Method of Gauss (MoG) are used to find the six orbital elements in conjunction with observational data taken over at least three separate nights, roughly equally-spaced in time.

Observations occurred at Sommers-Bausch Observatory (SBO-463) in Boulder, Colorado. We utilized the PlaneWave CDK20 optical tube telescope and STF-8300 for imaging. The PlaneWave CDK20 optical telescope has two fused silica mirrors: one at 7.5 in (191 mm) and one at 20 in (508 mm) [6]. It has a focal length of 3454 mm and focal ratio of F/6.8. The STF8300 uses the KAF-8300 CCD, with a total of 8.3 million pixels [6]. SkyX software was used for telescope navigation. Each night we took three sets of lights using the UV/IR cut-off filter with 10-minute intervals in between each series. We also acquired darks and flats for every night of observation. The darks were taken with the same exposure time as the lights. The apparent magnitude of 2015 HH10 shifted each night, which dictated our exposure times accordingly. Table 1 lists the dates and times for our four successful observations.
Astrometry was first performed on the acquired data from observation. AstroImageJ was used to detect 2015 HH10 across data from each night. The first task after every observation was to find 2015 HH10 throughout the series we took. We generated master darks and master flats. We then subtracted and divided our light series by the darks and flats to adjust for systematic error in the camera and telescope. In order to identify the movement of 2015 HH10 from the many stars in the frame, we cycled through the series and looked for movement. The best frame from each series was then uploaded into nova.astrometry.net to determine the transformations from pixel coordinates to celestial coordinates and to download the files containing the errors of these transformations. Once reopened in AstroImageJ, we selected our asteroid using the measurement tool and recorded the right ascension (RA) and declination (DEC) of our asteroid.
We next performed photometry on our data to determine the magnitude of 2015 HH10 using SAOImageDS9 and data from the AAVSO Photometric All Sky Survey (APASS). A trend line was generated from chosen star targets’ magnitudes and signals (average noise in the sky). The signal of the asteroid was input into the line of best fit to produce its magnitude. Astrometry and photometry results were formatted and sent to the Minor Planet Center for review and consideration.


To go from x and y pixel values to RA and DEC values, we must use a coordinate transformation. Least Squares Plate Reduction (LSPR) uses stars with known RAs and DECs to create a least squares fit for this transformation. LSPR is a statistical procedure that approximates the best fit for a set of data points by minimizing the sum of the residuals for the points from the curve. This method can be used to predict certain dependent variables. We used nova.astrometry.net to conduct LSPR on our images since the site allowed us to find the RAs and DECs of our asteroid during our observations. Table 2 lists the RA and DEC of 2015 HH10 across all four observations and individual series taken.
As previously stated, we utilized the Method of Gauss (MoG) and data from our last three observations to determine a preliminary orbit for 2015 HH10. There are numerous steps to MoG, beginning with finding an initial asteroid vector guess using the scalar equation of Lagrange an inputting Gaussian time intervals gathered from the time of observation. These Lagrange scalars are used to calculate an initial position magnitude. We can use the position magnitude and a truncated Taylor series expansion of r about the central value to determine the Lagrange coefficients, f and g. These Lagrange coefficients can be used to find the position and velocity vectors. After adjusting the times by accounting for the travel time of light, new Lagrange coefficients are found. This process is iterated to produce increasingly accurate values for the asteroid’s position and velocity vectors at the middle observation. The final position and velocity vectors are used as inputs in addition to the time of observation to find the orbital elements for 2015 HH10. The entire process requires data from three separate observations to predict the position at a fourth separate time.
To calculate the orbital elements, first the angular momentum (h) of 2015 HH10 was calculated at the middle observation time by taking the cross product of the position (r2) and velocity ( r2) vectors produced by MoG.

The semi-major axis (a) was then calculated by using the middle observation’s position and velocity.
Eccentricity (e) of the asteroid was calculated with the previously determined angular momentum (h) and the semi-major axis (a).

The true anomaly (ν) and the angular distance (U) from the ascending node to the asteroid were used to find the argument of perihelion.
Then the eccentric anomaly (E) was calculated.
The inclination (i) was calculated using the angular momentum (h).
The mean anomaly (M) was found using the eccentric anomaly (E).
The longitude of the ascending node (Ω) was calculated from inclination (i) and angular momentum (h).
Last of all, the time of perihelion (T) was determined using the current time and the mean anomaly (M).
To find the argument of perihelion (ω), the angular distance from the ascending node to the asteroid and true anomaly needed to be calculated first. We calculated angular distance from the ascending node to the asteroid (U) from the central position vector (r2), inclination (i), and longitude of the ascending node (Ω).
Constant k is the factor needed to convert from regular days to Gaussian days.This was calculated through our Python orbital determination code.
The true anomaly (ν) was calculated from eccentricity (e), semi-major axis (a), central position (r2) and velocity vectors ( r2), and angular momentum (h).






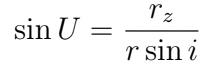

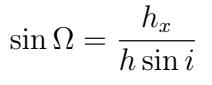


We compared our resulting ephemeris derived from the computed orbital elements to the online JPL Horizons database to determine our accuracy. We conducted Monte Carlo simulations to visualize the full range of values for each of our orbital elements, factoring in the RA and DEC uncertainty provided by nova.astrometry.net. For each Monte Carlo iteration, we chose RA and DEC values from Gaussian distributions with the mean as the average values of RA and DEC and the standard deviation as the uncertainty in RA and DEC. These values were run through the code to produce orbital elements. The results were plotted onto histograms, one for
each orbital element. We ran 100,000 total iterations of our Monte Carlo simulations.


To assess the consistency of our ephemeris generation program, we inputted orbital elements from the middle observation in addition to the date of the last observation to calculate the expected RA and DEC of the last observation. The expected RA and DEC was then compared to the actual observed value.



The Monte Carlo simulations produced orbital elements that had somewhat small error bars and values close enough to JPL Horizon’s predicted values that they fell well within those error bars, around one standard deviation away.
The generated ephemeris using data from three of our observations and the orbital elements we found using the MoG yielded RAs and DECs very close to the measured values even on the observation nights we didn’t use as our MoG input data. The errors between the MoG ephemeris values and the astrometry measurements are consistently lower than the JPL Horizon predictions (Table 3), sitting under 0.02% for all RAs and DECs. While JPL Horizon’s results
Figure 2. Orbit Visualization; 2015 HH10 is the blue orbit and Earth is the green orbit
Figure 5. Longitude of Ascending Nodes (DEG) (left), Argument of Perihelion (EG) (right)

are also relatively accurate, their percent errors are greater than what MoG produced. JPL Horizons has a particularly higher error in DEC calculations than RA. In general, the JPL Horizons database seems to less accurate, followed by the Monte Carlo simulations. All in all, our self-generated MoG ephemeris produces the most accurate predictions for the position of 2015 HH10 at a specific time. The minuscule percent errors, even for the observation night which was not included in our method of Gauss data, indicate a high level of accuracy in our methods and procedures. Certain complications arose throughout the process. When assessing data from observation one to calculate the position (r2) and velocity ( ̇r2) vectors, the r2 values did not converge, so the first observation was neglected for our final orbital determination. In addition, noise and star interference prevented the analysis of every series. In the third series of observation one, the asteroid was too faint to detect, preventing the AstroImageJ program from detecting it for proper measurements. In the second series of observation three, the asteroid passed in front of a star,
making it impossible to take measurements of its RA, DEC, and Source. More observations and more data would increase the accuracy of our orbital determination. Instead of just taking one set of orbital elements from a single observation date, it would be more precise to average the values of several sets of observations and use those averages to predict 2015 HH10’s future position.

We would like to thank Dr. Michael Dubson and Dr. Donovan Domingue, our professors, for guiding us through the conceptual foundation of our research and providing us support with the real-time application and creation of our project. We would also like to thank all the TAs, including Peter Lande, Jessica Dong, Mia Liang, and Grace Edwards for their help and patience with helping us debug our code, and supervising late-night observations. We’d like to recognize the University of Colorado Boulder for providing us with the facilities and technology to conduct our research at the Sommers-Bausch Observatory. Last, but not least, thank you to the Summer Science Program for providing us with this opportunity.
As wildfires burn millions of acres each year around the globe and have become more severe due to climate change, wildfire prevention is more important now than ever. Existing wildfire surveying techniques such as hotspotting and cold trailing require human interventions that can lead to dangerous situations or satellite imagery which does not provide real time data on hotspots. To address this problem, we propose a low-cost and effective integrated system of robots composed of an unmanned aerial vehicle (UAV, or drone) and unmanned ground vehicle (UGV, or rover) that autonomously cooperate and pathfind to detect and investigate hotspots. The UAV monitors a post-forest fire area from the air and uses aerial footage to create long-term direction for the UGV to inspect specific suspected hotspots. The UGV then follows the path to investigate each hotspot with centimeter-level accuracy. Testing of the pathfinding system with satellite imagery yielded highly accurate and consistent results necessary for high-precision autonomous navigation when investigating hotspots in dynamic environments.
Wildfires have an enormous human, environmental, and economic impact on society. In addition to lost human lives and massive carbon emissions, wildfires cost over $16 billion in damages to structures and fire management alone in the US in 2020 [1]. An essential part of wildfire prevention is identifying hotspots which are loose embers that stem out of a larger fire and light smaller pockets of fire. Currently, hotspotting relies on different techniques: watch towers, firefighters, satellite imagery, and aircraft. Watch towers can be expensive as they require around-the-clock staffing and only provide a limited detection range and angle of view. Firefighters can be deployed to detect hotspots and cold trails, which involves putting their hands in the ground to check for small pockets of embers after the wildfire has died down, but this procedure can lead to dangerous situations and requires extensive human resources. Satellite imagery, albeit precise for hotspot detection, cannot provide real-time data about these hotspots [2]. Finally, aircraft such as helicopters and planes can provide imagery for hotspot detection, but they require space to take off and are expensive to maintain and fly. The solution we investigate in this paper involves the integration of a UAV and UGV into a single autonomous system where the UAV finds a path between suspected hotspots and the UGV investigates them on the ground. The UGV also avoids undetected obstacles using our short-term correction algorithm. Previous research has demonstrated that UAV and UGV cooperative systems are useful for mapping environments and search and rescue [3], [4]. These systems, however, have not been specifically developed to address wildfire prevention through hotspot mapping.
In this paper, we propose a low-cost scalable system of UAVs (drones) and UGVs (rovers) to automate hotspotting. Using a protocol called LDSC (long-term direction, short-term correc
tion), a drone flies up and creates a long-term path for the rover based on aerial footage. The rover follows that path and avoids obstacles unidentified by the drone using short-term correction.
The system consists of three main components: a drone, a rover, and a ground station, as seen in Fig 1. All devices are connected through a server powered by Apache Kafka to which data and commands are sent [5]. The order of operations for the system is:

1) The drone lifts off, travels to its designated waypoint, and takes a photo of the area in which the rover will be moving.
2) The image is then sent to the ground station which creates a path using a modified pixel weighting A* pathfinding algorithm [6]. The path includes points of interest detected by the drone (suspected hotspots). Each pixel in the path is converted to GPS coordinates.
3) The GPS coordinates are then sent to the rover. The rover follows the path and adjusts to any obstacles overlooked by A* with an obstacle-avoidance algorithm.
In this paper, we built a drone and rover from scratch as shown in Fig. 2 and Fig. 3. As the drone and rover need to navigate autonomously, make intelligent decisions, and communicate with each other during navigation, the vehicles need to be computationally capable yet physically small. Seen in Fig. 2 and Fig. 3, both the drone and rover use a pixhawk 2.4.8 as the flight controller [7]. Attached companion computers allow for communication, autonomous
movement, and computationally heavy tasks. The drone uses a Raspberry Pi 4B+ to minimize weight, while the rover uses a Nvidia Jetson Nano for higher computational power [8], [9]. The vehicles are powered by lithium polymer (LiPo) batteries, and the companion computers are powered by separate USB battery packs. Both vehicles localize themselves using a GPS compatible with an APM serial port. The drone holds a gyroscopic camera gimbal with 9g servos and an Arducam Wide Angle lens camera [10]. The rover is driven by one motor in the back and a servo in the front to steer. It is also equipped with a SlamTec RPLIDAR A1 unit to provide a 2D map of surrounding obstacles [11].

All hardware components were purchased from common marketplaces for less than $2,000, shown in Table I. In total, half of the budget was spent on components usable for both the drone and the rover. A detailed list of components and their costs is available in the Github repository for this article.

The rover and drone use Python 2.7 and several Python libraries, including MAVProxy 1.8.46, DroneKit 2.9.2, OpenCV, and PyGeodesy 22.7.22 [12]–[15]. A startup script launches MAVProxy, which connects to the vehicles’ pixhawks. To begin navigation, DroneKit navigation commands are sent to MAVProxy. Computer vision is handled with OpenCV for camera calibration, the A* pathfinding algorithm, and capturing images, while PyGeodesy helps in the process of converting image pixels to GPS coordinates.

The drone, rover, and ground station all communicate using Apache Kafka. Each device has a consumer that listens to a topic on a server created on the ground station. Commands are sent by the producers as JSON serializables. After a consumer receives a command that is labeled for its device, it analyzes the command and executes its operations. The device then communicates to its respective producer to send any data back to the topic. Any number of devices can be added to this network using this framework, making the number of vehicles in the system scalable.
The drone, shown in Fig. 2, is a quadcopter designed to capture an aerial map of the target area and design a path between hotspots for the rover to follow. Using dronekit and waypoint finding, the drone autonomously flies to a target altitude and GPS coordinates, takes a photo using a gyroscopic camera mount, and sends that photo to the ground station for further processing.
1) Gyroscopic Camera Mount: To ensure accurate conversion of pixels into latitude and longitude coordinates, a 2-axis servo mount is used in conjunction with a mpu6050 to keep the camera facing the ground irrespective of the drone’s movement [16]. During the flight, the program starts a subprocess that perpetually adjusts the x and y-angles of the camera mount to compensate for tilting.
2) Camera Calibration: The drone uses a camera with a wide-angle lens to capture 2D images of the ground. Due to the innate characteristics of a wide-angle lens, optical barrel distortion causes objects in images to appear closer or further apart than they actually are. Distinct from the rest of the system, camera calibration was conducted using the OpenCV checkerboard test in order to undistort images and ensure accurate mapping [17]. Several images of a checkerboard were taken from different viewpoints
to estimate distortion coefficients and parameters of the camera. The mean reprojection error, a measure of the calibration’s accuracy, was 0.0459 pixels, well below the value of 1 that typically represents accurate estimations. Images were then able to be undistorted using the estimated distortion coefficients [18]. After taking the undistorted image, the drone waits until it is connected to the Apache Kafka server, converts the image into a bitmap/JSON serializable, and sends the image to the Kafka topic. The drone then returns to its starting waypoint and lands.

After the ground station’s Apache Kafka consumer receives the image from the drone, the ground station converts the image back into an OpenCV numpy array and performs the A* algorithm to create a path connecting the nodes of interest (i.e. the suspected hotspots). In a further embodiment of this research, existing thermal imaging techniques could be used to detect the exact location of the hotspots, but this example used aerial camera footage and manually selected nodes [19].
1) A*: A* is a search algorithm that finds the most efficient path between two nodes using a heuristic value (the distance to the end node) and the cost value of moving between each node. The algorithm is run on an image, representing each pixel as a node in the graph with up to eight neighboring nodes. Compared to other algorithms such as Dijkstra, A* is significantly faster. In this version, the cost (c) of moving between nodes is represented as a three-dimensional euclidean distance between the RGB values (each ranging from a value of 0 to 255) of both nodes shown in the equation below:
Weighting pixels based on color is used for the cost of the path, as it provides a way to determine changes in terrain using a visual camera which is more affordable than other sensors such as 3D LIDAR or stereo vision. Us-
ing color as the cost means that the path prioritizes roads and other areas of the map that have similar terrain for the rover to travel on.
Where f is the focal length, which was estimated during camera calibration. Third, the real-world distance dworldx and dworldy are calculated as shown in Fig. 4.

Figure 4. Diagram of pixel to GPS coordinate algorithm: the blue box represents the image plane, the green box represents the real world
2) Pixel to GPS Coordinates: The center of the image (χc, γc) is taken as the GPS drone location (αc, βc) where α is latitude and β is longitude. The altitude and drone location are known when the image is taken. The degree of the camera mount in relation to the compass of the drone is also known and is used to adjust the heading of the photo to face North. The algorithm to convert a single pixel to GPS coordinate works as follows.
First, the pixel distance between (χp, γp) and (χc, γc) is converted into meter based distance dimgx and dimgy as shown in Fig. 4. dimg is calculated by dividing the number of pixels between (χp, γp) and (χc, γc) by the resolution (pixels per meter) of the image.
Second, the magnification (M) of the image is calculated with the following formula: where odist is the height of the drone, and idist is the distance between the lens and the image sensor of the camera. idist is derived from the lens equation:
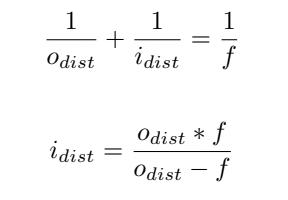
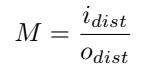
Finally, the latitude and longitude coordinates (αc, βc) of the pixel are calculated using the Vincenty formula from PyGeodesy which combines computational efficiency and accuracy up to a meter [20]. The NAD83 datum model of Earth’s surface was incorporated to to improve the formula’s accuracy.
The rover, shown in Fig. 3, is designed to follow the long-term path and avoid obstacles not seen by the drone. After receiving the GPS path from the ground station, the rover uses dronekit to autonomously travel to coordinates on the path representing the suspected hotspots.
1) Short-Term Correction: As the drone has only one camera and the A* algorithm relies on color, some obstacles on the path may be missed. GPS drift may also cause the rover to deviate from the path. To prevent collisions, an obstacle avoidance protocol based on the bug algorithm was implemented, enabling the rover to avoid objects in the short term before returning to the drone’s long-term path [21]. If the rover’s LIDAR detects an obstacle on the upcoming 2 meters of the path, the rover turns in the direction that brings it closer to the final destination and begins wall-following. Assuming that the final destination of the path is not located inside the obstacle, the rover returns to the long-term path when the path re-emerges from the obstacle. Because leaving the path is dangerous, the conservative design of this protocol ensures that the rover only

Tests were conducted on satellite images, but the methods were consistent with the steps outlined above except that the magnification was calculated using Google Earth scale factors.
ordinate information, as shown in the right image of Fig. 5. The GPS coordinates form a path that is nearly identical to the original pixel path in the left image of Fig. 5.
Figure 5. A* Path and GPS path comparison. Left: image of A* path run on satellite image with multiple hotspots as intermediary nodes. Path line thickened for ease of viewing. Right: Image of KML file containing GPA path generated from the path on the left shown in Google Earth. The point of each pin marks a point on the path.


First, a satellite image was selected to mimic a possible forest fire with dead trees and roads. Three target points labeled hotspots 1, 2, and 3, as seen in the left image of Fig. 5, were manually selected for the rover to investigate. The A* algorithm was then run to generate a path that connects each hotspot, starting with the start node and ending with hotspot 3. The resulting path is shown in the left image of Fig. 5. When there was a clear road, the algorithm tended to stay on it as shown in the first and third parts of the path colored in cyan and magenta. Also, in this test, the color of the brush and ground was difficult to differentiate, resulting in the path crossing the brush. However, it’s important to note that the rover would adjust during navigation using the short-term correction algorithm.
Next, the pixel to GPS coordinates algorithm was used to convert the pixel path into GPS coordinates. The coordinates were saved to a KML file, a file to share Google Earth co-
Figure 6. The A* results for the three paths tested: green circles represent start points and red circles represent end points

Figure 7. Xtrack Error from the rover following the three different paths
To test the rover’s accuracy in following the A* path, the xtrack error (i.e. the deviation from the desired GPS path in meters) was recorded

for three new paths of varying lengths and turns shown in Fig. 6. Each path was followed three times by the rover. Path 1 was the shortest and path 3 was the longest, and 400 to 800 data points were measured for each path. Fig. 7 shows the normalized distribution of xtrack errors for each path, and Table II summarizes the key results.
As seen in Fig. 7, the xtrack error ranges ±15 cm, with standard deviations below 5 cm across all paths. The small interval of xtrack error shows the rover’s ability to stick to its path consistently despite GPS drift and environmental factors. The fact that the standard deviation in Table II decreases as the path’s length increases reinforces the system’s consistency.
A system of unmanned drones and rovers cooperating together is an effective solution to investigate hotspots. Due to its low costs (below $2,000 for the entire system), the solution is scalable and could be used to complement human efforts and satellite and aerial surveillance. As seen in the results, the system can create a path from aerial footage, convert it into real-world coordinates, and guide the rover to accurately follow the path. The accuracy and consistency of the system indicate practical applications in high-precision autonomous navigation such as investigating hotspots in a dynamic environment. To view the code created for this project and the indepth parts list, please visit the GitHub link at: https://github.com/IRT-Drover/UAV-and-UGVAutonomous-Cooperation-for-Wildfire-Hotspot-Surveillance
Further embodiments of this research could include a drone with more sensors including a thermal camera to implement current imaging techniques to detect the hotspots from an aerial view. The pixel to coordinates algorithm’s accuracy could be improved by using a visual odometry library. The rover needs to be equipped with sensors to determine the severity of a hotspot to prioritize and give information to firefighting organizations. Finally,
further testing could be done to increase the number of drones and rovers in the system.
We would like to extend our thanks to the Pingry School for funding this project and providing us with useful resources and opportunities. We would also like to thank the past and current members of the Drover project at the Pingry School, in particular, Nicholas Meng, Ayush Basu, and Shaan Lehal. We would also like to thank Olivia Taylor for her support.
[1] M. Wibbenmeyer and A. McDarris, “Wildfires in the United States 101: Context and Consequences,” Resources for the Future, Jul. 30, 2021. https://www. rff.org/publications/explainers/wildfires-in-theunited-states-101-context-and-consequences/ [2] R. Allison, J. Johnston, G. Craig, and S. Jennings, “Airborne Optical and Thermal Remote Sensing for Wildfire Detection and Monitoring,” Sensors, vol. 16, no. 8, p. 1310, Aug. 2016, doi: 10.3390/s16081310. [3] J. Delmerico, E. Mueggler, J. Nitsch, and D. Scaramuzza, “Active Autonomous Aerial Exploration for Ground Robot Path Planning,” IEEE Robotics and Automation Letters, vol. 2, no. 2, pp. 664–671, Apr. 2017, doi: 10.1109/lra.2017.2651163.
[4] I. D. Miller, F. Cladera, T. Smith, C. J. Taylor, and V. Kumar, “Stronger Together: Air-Ground Robotic Collaboration Using Semantics,” arXiv:2206.14289 [cs], Jun. 2022, Accessed: Jul. 31, 2022. [Online]. Available: https://arxiv.org/abs/2206.14289#: :text=Stronger%20 Together%3A%20Air%2DGround%20Robotic%20 Collaboration%20Using%20Semantics
[5] “Apache Kafka,” Apache Kafka. [Online]. Available: https://kafka.apache.org/documentation/
[6] “Introduction to A*,” theory.stanford.edu. https //theory.stanford.edu/ amitp/GameProgramming/ AStarComparison.html (accessed Jul. 31, 2022).
[7] “Pixhawk Overview—Copter documentation,” ardupilot.org. https://ardupilot.org/copter/docs/common-pixhawk-overview.html
[8] R. P. (Trading) Ltd, “Raspberry Pi 4 Model B specifications,” Raspberry Pi. [Online]. Available: https:// www.raspberrypi.com/products/raspberry-pi-4-modelb/specifications/
[9] “Jetson Nano Developer Kit,” NVIDIA Developer, Mar. 06, 2019. [Online]. Available: https://developer.
nvidia.com/embedded/jetson-nano-developer-kit
[10] “USB Webcam Camera Modules,” Arducam. https://www.arducam.com/usb-board-cameras-uvcmodules-webcams/#wp-block-themeisle-blocks-advanced-columns-1d97200c (accessed Aug. 01, 2022).
[11] “RPLIDAR A1 Low Cost 360 Degree Laser Range Scanner Development Kit User Manual Model: A1M8,” 2016.
[12] “MAVProxy — MAVProxy documentation,” ardupilot.org. https://ardupilot.org/mavproxy/#: :text=MAVProxy%20is%20a%20fully%2Dfunctioning (accessed Jul. 31, 2022).
[13] ”Welcome to DroneKit-Python’s documentation!,” dronekit python.readthedocs.io. https://dronekit-python.readthedocs.io/en/latest/ (accessed Jul. 31, 2022).
[14] “OpenCV: OpenCV modules,” docs.opencv.org. https://docs.opencv.org/4.x/ (accessed Jul. 31, 2022).
[15] “PyGeodesy,” mrjean1.github.io. https://mrjean1. github.io/PyGeodesy/ (accessed Jul. 31, 2022).
[16] “MPU-6000 and MPU-6050 Register Map and Descriptions Revision 4.2 MPU-6000/MPU-6050 Register Map and Descriptions,” 2013.
[17] “OpenCV: Camera Calibration,” docs.opencv.org.
https://docs.opencv.org/4.x/dc/dbb/tutorial py calibration.html (accessed Jul. 31, 2022).
[18] “Reprojection error,” https://support.pix4d.com/. https://support.pix4d.com/hc/en-us/articles/202559369-Reprojection-error (accessed Jul. 31, 2022).
[19] A. Viseras, J. Marchal, M. Schaab, J. Pages, and L. Estivill, “Wildfire Monitoring and Hotspots Detection with Aerial Robots: Measurement Campaign and First Results.”

[20] C. V. www.movable-type.co.uk , “Vincenty solutions of geodesics on the ellipsoid in JavaScript — Movable Type Scripts,” www.movabletype.co.uk. http://www.movable-type.co.uk/scripts/latlong-vincenty.html (accessed Jul. 31, 2022).
[21] Howie Choset, K. M. Lynch, and S. Hutchinson, Principles of robot motion: theory, algoritms, and implementations. Cambridge, Mass. Bradford, 2005. Accessed: Jul. 31, 2022. [Online]. Available: https://www. scholars.northwestern.edu/en/publications/principles-of-robot-motion-theory-algorithms-and-implementations
In Drosophila melanogaster, Wiskott-Aldrich syndrome (WASP) family proteins play a critical role in many cellular processes involving reorganization of the F-actin cytoskeleton. Subfamily members Scar, Wash, WASp, and Whamy, have been implicated as essential during early Drosophila development. While studies have investigated the role of WASp and other isolated subfamily WAS proteins (Washout and Scar), the function of Whamy remains unclear. However, Whamy’s association with actin, membranes, and microfilaments during early embryogenesis suggests a role in the microfilament cytoskeleton. Since it has been established that WASP family proteins function as a connector between the cell membrane and Arp2/3 complexes to polymerize F-actin during cytoskeleton development, this study focused on elucidating the role of Whamy during cellularization and microfilament ring constriction by analyzing the mutant phenotype. Wildtype (WT) OreR embryos and mutant Whamy embryos were collected and stained with Neurotactin and Zipper antibodies to visualize cell membranes and myosin in the embryos during cellularization. Quantitative analysis of the microfilament rings showed a twofold increase in the number of microfilament rings in the mutant Whamy compared to WT, which suggests that the mutant protein induces cells to go through an additional cell cycle. Imaging of the Whamy microfilament rings showed that the rings undergo constriction at a much earlier stage than WT microfilament rings. These abnormalities in microfilament ring development during cellularization suggest that Whamy is necessary for normal development of the microfilament rings and that mutations in this protein may contribute to defects in cytoskeletal development.
Drosophila embryo during cellularization, stained with Neurotactin and Zipper

 by Ethan Boroditsky (V), Dr. John McLaughlin1 1Rutgers University, New Brunswick, NJ
by Ethan Boroditsky (V), Dr. John McLaughlin1 1Rutgers University, New Brunswick, NJ
Every year, tens of billions of dollars are lost in harvest from the Fusarium graminearum fungus attacking and killing critical agricultural plants such as corn, wheat, barley. As it turns out, this fungus uses mycotoxins to kill these plants. Our goal is to better understand the mycotoxin’s mechanism of action. In this study, we used a small unicellular algae called Chlamydomonas reinhardtii as our model organism, because it allows us to better understand and experiment with this toxin. Our study took advantage of a comprehensive genome-wide mutant library for Chlamydomonas. Such a library allows for the performance of screens that simulate identical conditions for an organism with every single knockout mutant simultaneously. We looked at differences in growth as a phenotype representative of fitness. During this project, we wrote a Python program utilizing various libraries to automate the process of searching the internet for hundreds of unique accession codes that correspond to homologous mutants of interest in Arabidopsis thaliana. This allowed us to learn more about the molecular mechanism and cell components the genes were primarily involved in, while also beginning to make the jump to a higher organism.
Chlamydomonas reinhardtii is the optimal model organism for studying the plant stress response in the chloroplast for many reasons. Chlamydomonas is a eukaryotic single-cell organism that performs photosynthesis. Most importantly, Chlamydomonas can be transformed and mutated, and as of 2020, there is a genome-wide mutant library that is publicly available. This allows for the screening of the entire Chlamydomonas genome. In this study, mutants were screened with a mycotoxin believed to induce plant stress. Sensitivity was measured by assessing mutants’ growth and assigning a representative “fitness score.” It was hypothesized that the key genes in the plant stress response mechanism would be the ones with the lowest fitness scores in the respective mutants. Finally, we were able to utilize a gene ontology (GO) analysis to identify which molecular mechanism and cell components the genes were primarily involved in. Throughout the study, a few specific genes and proteins of interest were investigated individually, including D1, VIPP2, and MARS1. These three proteins are involved in the removal and repair of degradation products and aggregates that form as a result of translation inhibition, which is induced by plant stress.
This study was designed to elucidate the mechanisms through which mycotoxin-induced stress is dealt with in plants. The GO analysis shows what cellular components and molecular pathways the mutants are involved in [1]. The mutant library that we used was printed in quad format on 9 48x32 plates. This library also had a comprehensive spreadsheet in a 36x384 (columns x rows) format containing unique identification codes that corresponded to each mutant. To convert from the first format to the second, Python code was written using the Pandas library. The mutants were printed to each plate from subsets of 4x384 sheets. Within each subset, each row was printed as a quad, filling the 48x32 plates horizontally, two rows at a time. To reverse engineer this process, I took the 4x384 groups and broke them into 48x32 plates. As shown in Figure 1, each of the 9 plates can be broken down into smaller groups of two rows. Each of these two rows is filled by going down the 4x384 sheet, putting the first two mutants into the top row, and the remaining two mutants in the bottom row. Repeating this process for each 4x384 group generates the 9 separate mutant plates. The core of the python code for
Figure 1. Within each subset, each row was printed as a quad, as shown in (a), while we need to populate rows horizontally as shown in (b)

Figure 2. The graph shows the fold change of VIPP2 and MARS1 expression after treatment with DON and Tcin after 1 and 24 hours. This data was gathered through RT-qPCR

this part of the project is shown in Appendix 1. After organizing the 9 plates, we were finally able to work with our physical library. The library was screened twice, once with Tcin and once with Lincomycin. After the screening, 156 mutants were identified as having the lowest fitness scores. These scores were assigned solely based off phenotypic growth. The phenotyping was done using the Balony software. Before performing the GO analysis, each of the 156 mutants had to be converted from LMJ format into another format which the Panther classification software could interpret. Through trial and error, we found that TAIR accession codes would be best for this task, especially since they represent the orthologs of a higher organism, bringing us closer to experimenting with larger plants. Scouring the web for TAIR codes would be a long and menial task without automation. To automate the process, we wrote a Python program. As shown in Appendix 2, the program follows a path of two URLs, the first converting from LMJ to Cre format, and the second from Cre to TAIR format. While the mutant library is known as “genome-wide,” it only encompassed about 25% of the Chlamydomonas genome. This is because the entire genome is still being mapped out to this day. Not every gene in the Chlamydomonas genome that is mapped has a defined function, so only about 62 of 156 mutants
were found to have an Arabidopsis ortholog. In related studies, we identified Vipp2, Mars1, and D1 as genes of interest in the mycotoxin stress response [3][4]. We performed a RT-qPCR on the mRNA, the results of which are shown in Figure 2, measuring the expression of Vipp2 and Mars1 to confirm whether the two genes were involved in the stress response. We used wildtype Chlamydomonas and treated the samples with DON and Tcin, two types of mycotoxins, comparing treatment times of 1 and 24 hours. For our study of D1, we first treated samples of Chlamydomonas with DON. Following treatment, the samples were grown for two days, after which the protein was harvested. As shown in Figure 3, going from left to right, the concentration of DON treatment increased. The first and last lanes are ladder lanes. To quantify the immunoblot, we used an Odyssey machine. This tool measures the total amount of fluorescence emitted in a given area. In each lane of the blot, we then normalized the quantification to better understand the change in D1 quantity.

This study yielded findings that have brought us closer to achieving the goal of identifying genes in higher organisms that are involved in plant stress response. We found significant common-
Figure 3. Western immunoblot with increasing DON concentration from left to right. The quantity of DON respectively decreases, corroborating the idea that toxic stress degrades DON. The first and last lanes are Magic Marker ladders. Contrary to expectation, no degredation products of D1 are visible in the blot.
alities in some of the mutants, suggesting some of the potential pathways involved. The results of the GO analysis, which are displayed in Figure 5, reveal that of the 28.6% of mutants implicated in binding, two thirds were involved in cyclic compound binding. A cyclic compound is a molecule whose atomic structure is organized in the shape of a ring. Lincomycin and Tcin are both cyclic compounds, whose structures are shown in Figure 4. This suggests that the mutants we identified in this category are likely responsible for directly binding to and detoxifying the mycotoxins. In the next stages of our research, we will look more into the genes that we inhibited in the sensitive mutants. Understanding exact function of their respective proteins may hold the key to understanding how to improve plant resistance to DON and Tcin. One of the more surprising findings was that only 3 out of the 156 sensitive mutants showed sensitivity to both DON and lincomycin. Lincomycin is known to bind
in chloroplast-to-nucleus signaling. Therefore, our results are consistent with what was expected. D1 is a well-known Photosystem II protein that is highly sensitive to stressors such as high light [2]. In this study, we are propagating D1’s known sensitivity to phototoxic stress to include mycotoxic stress. D1 is known to be degraded as a result of light stress [2]. As shown in Figure 3, our blot is consistent with this, because the bands growth fainter as the DON concentration is increased. This is indicative of the degradation of D1. In this blot, we also were hoping to see degradation products of D1 below the band. However, no degradation products appear to be present below the band. The explanation for this may be that the treatment lasted too long. In future studies, we plan to sample the protein at earlier points after toxin treatment to look for aggregates and for D1 protein changes because it is likely given the concentrations we used, the cells can adapt and overcome damage by 2
Figure 4. The molecular structures above represent Tcin and Lincomycin respectively. Both are cyclic molecules.

to the ribosome and inhibit or alter translation. The lack of overlap indicates that mycotoxins act differently on the cell than lincomycin, meaning that mycotoxins act elsewhere in the cell.
According to Figure 2, 1 hour after treatment with DON and Tcin, Vipp2 and Mars1 are both expressed significantly more than after 24 hours. After 24 hours, the cell is likely closer to returning to homeostasis. Increase in expression is a clear indicator of Vipp2’s and Mars1’s involvement in handling mycotoxin-induced stress. Although the fold increase is thousands of times lower in Mars1 and Vipp2, that does not mean that Mars1 is any less significant in stress response. Since Vipp2 is a membrane protein, it must be present in greater quantity to uniformly and densely cover the membrane. Mars1, however, is a kinase involved
Figure 5. This pie chart represents the results of the GO analysis. It displays the individual molecular functions of the Arabidopsis ortholog mutants that were identified. 47.6% of mutants are involved in catalyic activity, while 28.6 % are involved in binding.

days in. The degradation products we had hoped to see may appear at shorter periods of time.


Here we report the early stage of the mycotoxin pathway study. Our final goal is to understand the genes that are involved in more significant agricultural organisms that contribute to the human diet. After we identify key genes in organisms such as Chlamydomonas, the research can move on to higher organisms. In the future, we plan to focus on the Arabidopsis orthologs involved in cyclic binding that we identified through the GO analysis. We will study their functions and discover which cellular component they are involved in and identify whether they interact with proteins of interest such as D1, VIPP2, AND MARS1. We plan to run shorter time intervals for harvesting D1 protein after treatment, to confirm the degradation of D1 from mycotoxic stress. As the Chlamydomonas library
is updated, we will continue performing GO analyses to potentially identify new Arabidopsis orthologs as well. With the 156 mutants that were identified, we plan to create double knockouts, so that we can begin to understand the protein-protein interactions going on in the cells.
[1] Gene Ontology, C., The Gene Ontology resource: enriching Gold mine. Nucleic Acids Res, 2021. 49(D1): p. D325-D334.
[2] Llamas, E. and P. Pulido, A proteostasis network safeguards the chloroplast proteome. Essays in Biochemistry, 2022.
[3] Perlaza, K., et al., The Mars1 kinase confers photoprotection through signaling in the chloroplast unfolded protein response. Elife, 2019. 8.
[4] Theis, J., et al., VIPP2 interacts with VIPP1 and HSP22E/F at chloroplast membranes and modulates a retrograde signal for HSP22E/F gene expression. Plant, Cell & Environment, 2020. 43(5): p. 1212-1229.
There is a serious public concern about the environmental contamination of soil and water with per- and polyfluoroalkyl substances (PFAS). These compounds are manmade chemicals and are considered to be toxic and persistent in the environment [1]. PFOA (Perfluorooctanoic Acid) and PFOS (Perfluorooctanesulfonic Acid) are part of the vast PFAS group of chemicals and most commonly occurring PFAS pollutants. In this study the sonochemical degradation technology was used to remove PFOA and PFOS from water. The destruction of PFOA and PFOS mixtures in ultrasonic reactor was studied at ambient temperature and in the air atmosphere at various sonication treatment times. The destruction efficiencies of PFOS and PFOA were demonstrated by direct measurement of PFOS and PFOA as well as the defluorination of PFOA and PFOS by the detection of fluoride ions in the water under treatment.
PFOA and PFOS are perfluorooctanoic acid and perfluorooctanesulfonic acid respectively (Figure 1) and are part of the group called PFAS (polyfluoroalkyl substances). PFAS do not exist in nature, they are man-made chemicals. The sources of PFAS in the environment, particularly in ground and surface water, include the firefighting foams, discharge from the manufacturing industries, and consumer goods such as coated fabrics and food packaging. Due to their unique chemistry (C-F bond is the shortest and strongest bond in nature), PFAS are environmentally persistent and bioaccumulative meaning that they are very stable and cannot degrade naturally. Because of its potential carcinogenic effect, PFAS are considered to be potential hazards to human health. The United States EPA (Environmental Protection Agency) recently announced that the PFAS health advisory limit is 0.004 nanograms/ liter in contrast to the previous limit of 70 nanograms/liter which calls for new more advanced remediation technologies which are able to reduce PFAS concentrations in the environment.

Sonication or, in other words, the use of sound energy (ultrasound), in environmental engineering is found to be very promising remediation technique for treatment of contaminated soils and waste waters. Since no toxic chemicals are used or produced during this type of treatment it is considered to be environmentally friendly. Among other benefits are high efficiency and a low cost. In this study the sonochemical degradation technology was used to remove PFAS from water.
PFOA (>98.0%) was purchased from TCI America. Potassium perfluorooctanesulfonate (K PFOS) (≥98.0%) was purchased from Sigma-Aldrich.
Standards of PFOA (>98%), K-PFOS (>98%) and EPA 533 PFAS standards, were obtained from Wellington Laboratories. Milli-Q water (18.2 MΩ·cm) was used in all experiments and analyses. A high-frequency ultrasonic bath reactor equipped with a 700 kHz plate transducer provided by PCT systems was used for experimental work. The detailed reactor set up is described elsewhere [2,3]. The total maximum power applied was 450 W. Experiments were conducted at ambient temperature (25±2oC) in the air atmosphere in a closed system to avoid any evaporation.
The experiments were performed as follows: The desired concentration of PFOA and PFOS mixtures were prepared in Milli-Q water. For testing, 10 Liters of the above prepared solution were placed in the bath reactor for the following ultrasonic treatment. The typical ultrasonic treatment run was 5 hours. For further analyses, duplicate five mL samples were collected before the experiment and every thirty minutes over the treatment time (300 minutes). These samples were further analyzed separately for PFAS and fluoride concentrations. The only known method which allows quantifying individual PFAS destruction is LC/MS/ MS. Therefore, the collected samples were analyzed for PFOA and PFOS concentrations according to the EPA 8327 [4] and EPA 533 methods [5] using liquid chromatography with tandem mass spectrometry (Agilent 6470 Triple Quadrupole LC/MS System [6, 7]). Prior to the LC/MS analysis, all collected water samples were diluted with methanol to have a concentration below 100 ppb and then filtered.
A fluoride-ion selective electrode (Thermo-Scientific, F-ISE) was used to follow the formation of inorganic fluoride ions in solution. Collected water samples were diluted with TISAB II (1:1 ratio) for the analysis.
Figure 1. Molecular structure of PFOA (left) and PFOS (right)
The destruction of PFOA and PFOS mixture in water in ultrasonic reactors was studied at various sonication treatment times. The normalized concentrations of PFOS and PFOA as function of the treatment time for a mixture containing the initial concentrations of PFOS and PFOA of 3.7µM and 6µM, respectively, were determined. The destruction efficiencies of PFOS and PFOA in percentages are demonstrated in Figure 2. As seen in Fig 2, the decomposition is sonication time dependable and at the end of the five hours of continuous treatment, 99% decomposition of PFOA and almost 96% decomposition of PFOS were observed.
Figure 2. PFOA and PFOS Removal as a function of treatment time
collected water samples. The concentrations of released fluoride ions into solution after treatment of the described above mixture of PFOA and PFOS were determined. The fluoride concentration in the reactor increases with the time of treatment and maximum achieved concentration after treatment was 112 µM. If we assume that the complete defluorination of both PFOA and PFOS occurs and all fluorine atoms are released into a solution, the anticipated concentration yield of fluoride ions in 10 L of treated solution can be calculated. Knowing that PFOS (C8HF17O3S, Molar Mass=500.13 g/ mol) molecule contained 64.6% fluorine atoms and PFOA (C8HF15O2, Molar Mass=414.07 g/ mol) molecule contained 68.8% fluorine atoms, for the given initial concentrations ([PFOS] o=3.7µM and [PFOA]o=6µM) the resulting total concentration of fluoride ions should be approximately 152 µM. Figure 3 shows the percentage of defluorination achieved in the experiment. There is about 26 % difference (152 µM versus 112 µM) between theoretical fluoride yield and observed in the current experiment. This difference can be attributed to the insufficient sonication time and based on the trend line (Figure 3) could be potentially achieved. However, the untreated PFAS (combined PFOA and PFOS) left after five hours of treatment can be accountable for only a tiny amount of “missing” fluorine atoms (about 9%). This reasonably leads to the possibility of existence of other fluorine containing by-products. According to the previous studies on the decomposition of PFAS by different technologies [8-5] there is a possibility of a complete destruction (or so called complete mineralization) when the perfluoroalkyl chain of the PFAS molecule is completely defluorinated. The anticipated byproducts formed in this case are exclusively carbon dioxide, carbon oxide or inorganic carbon, and hydrogen fluoride. Depending on the PFAS treatment/destructive techniques, another alternative is a partial defluorination of PFAS molecules leading to the potential formation of by products such as “short chain” hydrocarbons in addition to CO2 and CO. In order to follow the defluorination path of the decomposition, the released fluoride ions were monitored in
Figure 3. Percentage of defluorination as a function of treatment time


This study was focused on the sonochemical decomposition of the mixture of PFOA and PFOS and its relation to the treatment time. The experiments were conducted in the ultrasonic batch reactor at ambient temperature and in the air atmosphere. After five hours of treatment under experimental conditions, 99% decomposition of PFOA and almost 96% decomposition of PFOS were achieved. The formation of fluoride ions in treated water was detected and 74 % of defluorination of PFAS (PFOS + PFOA) was observed. However, incomplete defluorination leads us to believe in the existence of other fluorine-containing by-products which will be the subject of future study.
I would like to thank New Jersey Institute of Technology and its High School Summer Research Internship Program (HSSRI) for the opportunity to perform research over the summer. I would like to express my sincere gratitude to my advisor Professor Jay Meegoda and my laboratory mentor, doctoral candidate Jitendra Kewalramani, for providing me the unique opportunity to join and work with their wonderful research group. I would also like to thank the whole research team and my fellow HSSRI students for creating a friendly lab atmosphere and for all the help.
[1] R.C. Buck, J.Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. de Voogt, A. A. Jensen, K. Kannan, S.A. Mabury, S. PJ. Van Leeuwen, “Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and Origin, Integrated Environmental Assessment and Management”, 7 (2011) number 4, 513541.
[2] J. A. Kewalramani, B. Wang, R.W. Marsh, J. N. Meegoda, L. Rodriguez, “Coupled high and low-frequency ultrasound remediation of PFAS-contaminated soils”, Ultrasonic Sonochemistry 88 2022, 106063.
[3] J. A. Kewalramani, R.W. Marsh, D. Prajapati, J. N. Meegoda, “Sonochemical degradation of PFAS and PFOA in Concentrated Waste: Impact of Power Density and Initial Concentration,” submitted to Ultrasonic Sonochemistry, 2022
[4] SW-846 Test Method 8327: Per- and Polyfluorinated Substances (PFAS) by Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS), Hazardous Waste Test Methods, United States Environmental Protection Agency, https://epa.gov/system/files/documents/2021-07/8327.pdf

[5] Method 533: Determination of Per- and Polyfluoroal-
kyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry, United States Environmental Protection Agency, Office of Ground Water and Drinking Water, https://www.epa.gov/sites/default/files/2019-12/documents/method-533-815b19020.pdf
[6] Analysis of Per/Polyfluoroalkyl substances in Water Using Agilent 6470 Triple Quadrupole LC/MS, Agilent Technologies, Application Note 5991-7851EN, 2017, https://www.agilent.com/cs/library/applications/5991-7951EN.pdf
[7] EPA Method 533 for Analysis of Per/Polyfluoroalkyl substances in Drinking Water Using Agilent 6470 Triple Quadrupole LC/MS, Agilent Technologies, Application Note 5991-7863EN, 2020, https://www.agilent.com/ cs/library/applications/5991-7863EN.pdf
[8] R. K. Singh, S.Fernando, S. F. Baygi, N. Multari, S. M. Thagard, T. M. Holsen, “Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma based water treatment process”, Environ. Sci. Technol. 53 (2019) 2731-2738
[9] H. Moriwaki, Y. Takagi, M. Tanaka, K. Tsuruho, K. Okitsu, Y. Maeda, “Sonochemical Decomposition of perfluorooctane sulfonate and perfluorooctanoic acid”, Environ. Sci. Technol. 39 (2005) 3388-3392.
[10] J. Horst, J. McDonough, I. Ross, E. Houtz, “Understanding and managing the potential by-products of PFAS destruction,” Groundwater Monitoring & Remediation. 2020, National Ground Water Association
[11] S. Stockenhuber, N. Weber, L. Dixon, J. Lucas, C. Grimison, M. Bennett, M. Stockenhuber, J. Mackie, E. Kennedy, “Thermal degradation of perfluorooctanoic acid (PFOA)”, 16th International Conference on Environmental Science and Technology, 2019.
How do menstrual stigmas, period shaming, and poverty affect Afghan girls? In this paper, I found that many misconceptions are formed since there is limited sex education before menarche, which often harms girls’ physical health. I also discovered that many girls leave school because essential period hygiene management products and toilet facilities are not in schools. Through further research, I found that Afghanistan’s poor healthcare system adversely affects women’s access to medical care. In addition, I also discovered that the Taliban’s women’s rights violations harm girls’ and women’s livelihoods. Lastly, I found that girls in rural communities have difficulty accessing period products stocked in city shops since they are scarce and expensive. Afghanistan has many cultural and economic struggles, making the menstruation experience for girls and women difficult. The following recommendations are to have more doctors practicing in rural communities, raise the consent age for women to 18 years old, have menstrual hygiene products accessible in all public restrooms, increase government spending on education, and allow women to leave their homes unaccompanied and without permission if they are doctors or seeking medical attention, and are meant to combat many of Afghanistan’s and the Afghan people’s problems. Women make up 18.2% of Afghanistan’s illiterate population, and this percentage will keep rising until Afghanistan implements the suggested modifications to make it so that girls feel safe attending school while on their periods.
In Afghanistan, more than fifty percent of girls have menarche without knowledge, expectations, or an understanding of menstruation [52]. An April 2021 cross-sectional study, which surveyed 768 girls between the ages of 11 and 18 in the city of Herat, Afghanistan, confirmed this, concluding that over 60.8% of girls did not know what menstruation was prior to menarche [42]. Furthermore, ten to thirty percent of Afghan girls are absent from class each day because schools do not have the proper hygiene facilities for menstruating, such as separate toilets for girls, toilet paper, or waste paper baskets [52]. These numbers show that menstruation hinders the education of young Afghan girls.
In this paper, I seek to answer the question, how do period shaming, the stigmas around menstruation, and poverty directly harm Afghan girls? I argue that the unwillingness of parents and educators to provide pre-pubescent and menstruating girls and women with the proper education about human physiology, products for menstruation hygiene management, toilet facilities, and effective methods to manage pain when menstruating is among the principal causes of low literacy and severe gynecological conditions among Afghan females. With 7.2 million girls and women in Afghanistan, out of a total population of 39.6 million suffering from illiteracy, Afghan parents, doctors, and educators need to change their attitudes toward menstruation and make the appropriate changes, such as providing free menstrual products and sex education, to ensure menstruating girls are comfortable at school [58].
In Afghanistan, women must pay 4 USD for a single sanitary napkin, while the average salary of a teacher in Afghanistan is 672.81 USD a month [27]. Also, locating sanitary pads in city supermarkets is difficult since they are rarely seen and often hidden from patrons [20, 56]. These numbers reflect Afghanistan’s “period poverty,” meaning limited access to essential menstrual items, a lack of school hygienic facilities, and period shaming prevalence in society.
One cause of period shaming, “period poverty,” and its stigmas is that sexual education is unacceptable in deeply conservative Afghanistan; in fact, the mere mention of genital parts
or human reproduction is considered inappropriate. Unsurprisingly, young schoolgirls’ lack of knowledge of menstruation leads to poor hygiene practices and inaccurate beliefs about menstrual products. For instance, in Afghanistan, it is believed that washing oneself during menstruation can lead to infertility, but unbeknownst to them, the girls are in more danger of infection or skin irritation due to a lack of cleanliness [59]. Afghanistan’s cultural beliefs about female chastity and myths surrounding “virginity” also harm girls during their periods. Women and girls almost exclusively use pads as they believe tampons could potentially break their hymen, resulting in them not bleeding on their wedding night, which is a grave matter in conservative patriarchal cultures because it puts into question the woman’s sexual innocence [44]. Clearly, with the proper education about their bodies, the girls would be safe from falsehoods their parents, primarily their mothers passed on to them.
In addition, many Afghan girls must be absent from or leave school entirely simply because they are menstruating. A 2020 CARE study found that such disruptions in the young girls’ education cause approximately 30% of Afghan girls to develop depression or anxiety [12, 29]. This is made worse by the refusal of the Taliban, who are currently in charge of Afghanistan, to allow girls to attend secondary school [15].
In August of 2021, the United States evacuated Kabul, Afghanistan, ending twenty years of warfare [9]. The United States’ withdrawal from Afghanistan resulted in a catastrophic upheaval in the lives of Afghan women and girls, particularly in education, the workplace, and access to health care [60]. “Period poverty,” also, predictably, got worse. Companies like Safepad, which created “a reusable sanitary pad designed to provide a safe and infection-free experience;” the CDC’s WASH program, a global program that saves lives by improving access the adequate water, sanitation, and hygiene, through long-term prevention and control measures; and UNICEF, are striving to provide supplies, relief, and support. However, with the Taliban controlling Afghanistan, “period poverty” and stigmas can and will only worsen [11, 49, 60].
The Taliban, an Islamic fundamentalist group, first took control of Afghanistan in 2001 and has misogynistic behavior closely related to peri-
od stigmatization, period poverty, period shame, and general neglect of women’s health in Afghanistan. The group’s beliefs support the corrupt inferiority of women and girls, so girls and women in Taliban-controlled Afghanistan are subjected to unfair misogynistic treatment that is dangerous to their health and human dignity. With the Taliban currently in control, many new rules restrict women’s rights and access to healthcare. Generally, according to the UNFPA, Afghanistan does not get support from other countries, making life worse for Afghan girls and women under the Taliban regime [28].
Women across the globe are facing period stigma and poverty due to archaic cultural b eliefs, a lack of education, and limited access to menstrual hygiene products. In this section, I will provide examples to show the prevalence of period stigmatization and its harmful consequences for girls and women.
A particularly compelling example is India, a country with a long history of menstruation inequity. Sixty kilometers outside of New Delhi lies the Hapur District, and in Zehtabchi’s 2018 documentary, Period. End of Sentence, multiple Indian girls and women from the district were interviewed regarding their menstruation experiences. Many of the women interviewed had limited knowledge about menstruation. One older woman stated, “that is something only God knows,” when asked what occurs during menstruation [44, 01:22-01:33]. Cultural taboos that associate menstruation with impurity and evil also feed into India’s poor sex education. For instance, in India, it is believed that when a menstruating female touches a cow, it becomes infertile, which is, obviously, scientifically incorrect [4]. Likewise, with approximately 94% of the world’s Hindu population residing in India, Hinduism profoundly influences Indian culture and policies, including menstrual rights. Since it is “believed that menstruating women are unhygienic and unclean and hence …[everything they] handle can get contaminated,” Hinduism forbids women from participating in daily activities like praying or cooking while they are menstruating [4]. Likewise, women are also forbidden from tak-
ing a bath at the beginning of their period because menstrual blood would “pollute” the pure water [4]. Furthermore, Indian girls are also advised against exercising because exercise is thought to worsen period pain, which is medically incorrect [4]. Not surprisingly, most Indian schools lack proper private bathroom facilities, which causes many girls to drop out of school simply because they are menstruating [44, 02:41-03:28].
Like in many countries around the world, Indian women and girls are plagued by a lack of accessible and affordable menstrual products, so “girls see that they’re bleeding and use whatever cloth they can find;” but once entrepreneur Arunachalam Muruganantham discovered that menstruation was a key reason for women’s execution from full public participation in India, he built an inexpensive sanitary napkin machine [44, 06:34-07:21]. His “low-cost sanitary napkin machine” permitted women to make sanitary napkins using natural materials within their home, taught men and women about pads, and created jobs for women, granting them more respect within their households [44, 08:23-10:04].
Egypt is another country where shaming girls for their periods and misinformation about menstruation is a big problem. When menstrual hygiene products are purchased, convenience store clerks typically wrap sanitary napkins in newspapers because they are embarrassed to be seen with the product. Additionally, young Egyptian women are prevented from using tampons due to old myths and prejudices that it is thought to take a girl’s virginity or rupture the hymen, similar to Afghan beliefs [27]. Since sex education is the responsibility of parents and the subject is described as “shameful” in the Egyptian language, misinformation, like the idea that tampons soil virginity, can often spread [48].
Even in a progressive country like South Korea, discussing menstruation is usually avoided. For instance, when Women’s Health Magazine interviewed 31-year-old, Seungmee from South Korea, she stated that “we don’t see these things [like sex-ed videos] in Korea” [27]. She also shared that when she moved to Canada, she was surprised by the openness surrounding sexuality and tampon dispensers in public bathrooms, which influenced her mother to be more open to discussing the menstruation cycle [27]. Beyond Seungmee’s
experiences surrounding period shaming and lack of education, South Korea also has a long history surrounding “period poverty.” As a result of companies like Yuhan-Kimberly raising their pad prices and creating the “insole girls1” in 2016, the South Korean government was forced to allow the sale of menstrual cups, which were previously prohibited, in 2017, and to provide free menstrual products in 10 public venues in 2018 [14]. Thus, charities and non-profits were encouraged to distribute free menstrual products for girls. In addition, South Korea started offering unpaid menstrual leave in 2001, allowing women to take one to two days off each month due to menstruation; however, the regulation was altered in 2003 [14, 36]. The altered law required women to request menstrual leave, ultimately leading to workplace inequity and discouragement of women from taking advantage of the law [14].
Like many countries in the Global South, women in the United States also suffer from “period poverty,” menstrual shaming, and stigmas. Incarcerated individuals, students, transgender and nonbinary individuals, as well as low-income and homeless women and girls, struggle with “period poverty” in the United States mainly because of the levying of sales tax on menstrual products, formally known as the “tampon tax” [53]. The “tampon tax” is controversial in the United States because other goods like groceries and medicines, which “are considered non-negotiable necessities,” are exempt from such a tax [53]. Since menstrual products are necessary, they should also be tax-exempt. Consequently, a 2019 study explored that two-thirds of low-income American girls and women were struggling to afford tampons or pads, and “more than one in five women said they had this problem every month” [10]. The same study confirmed that impoverished women and girls were forced to utilize cloth, diapers, or toilet paper from public facilities to maintain their dignity while menstruating, similar to women and girls in India. Lastly, beyond aggressive taxes on menstrual products and “period poverty,” American sex education gives girls a negative view of menstruation, contributing to body shaming, self-objectification, and other mental health illnesses [55]. Nonetheless, the United States is making significant progress in creating laws and policies protecting girls and women during menstru-
ation. In the last ten years, the state and federal governments have passed 62 menstruation equity laws, with New York and Illinois passing the most. These laws include eradicating menstrual tax, making menstrual products more accessible in schools, prisons, and shelters, and transparency about the safety of menstruation products by disclosing ingredients [35].
The Taliban is making Afghan women and girls’ lives worse. Secondary school girls are no longer permitted to attend school, and many other new rules restrict women’s rights and access to healthcare. For instance, under Taliban edicts, women and girls are forbidden from showering in public baths, even on women-only days, or attending social activities, like weddings, while menstruating [12]. Likewise, new guidelines state that male doctors are permitted to touch female patients only above clothing, and women are not educated on health care practices, keeping medicine a primarily male-dominated territory [34]. Women gynecologists are permitted to treat women, but their numbers are few, and pregnant women are often hesitant to leave their homes. It is predicted that more healthcare restrictions will be placed on women and girls, making it more difficult to receive proper medical care. According to the UNFPA, it is suspected that if Afghanistan does not get support from other countries, there could potentially be 4.8 million unplanned pregnancies, 51,000 maternal deaths, and two times as many people will not have access to family planning facilities between 2021 and 2025 [28]. Furthermore, the Taliban are also increasing child and forced marriages as militants marry young girls, leading to more child pregnancies and, eventually, a higher maternal mortality rate [28]. The Taliban is also making it difficult for female healthcare workers to work because those who are married are not allowed to leave their house without their husband’s permission or accompaniment, putting Afghanistan in a severe shortage of medical professionals, with 4.6 doctors per 10,000 Afghans [2, 7].
The mistreatment of Afghan girls during their adolescent years goes beyond stifling their education. Girls that leave school because of the inability to manage menstruation will face issues with employment later in life; regardless, girls who do complete schooling with bachelor’s and master’s degrees still find employment difficult. In Afghanistan, many educated women are unemployed, in irrelevant jobs, or working part-time jobs [1, 51]. High unemployment, especially among educated women, has worsened since the Taliban regained control. For example, 900,000 Afghans lost their jobs, mostly women, because the Taliban forced women out of work [43]. Due to the Taliban’s enforcement of strict policies, such as mandating the burqa in public and requiring women to be accompanied by a male when going outside, many women are highly discouraged from working [39]. These new mandates are damaging the livelihood of many women, mainly because after many years of war, many women have been left widowed and must be their family’s “sole breadwinner” [39]. These new mandates enforced by the Taliban are blatant attacks on Afghan women’s rights, and, besides restricting their education, it also threatens the future livelihood of Afghan girls.
As of 2020, Afghan women have an average life expectancy of 67 years compared to a life expectancy of 80 years in the United States and 78 years in Iran [30, 57]. Due to the lack of medical care provided to Afghan women and girls as a result of the renewed Taliban takeover, they often have fatal reproductive lives. Female adolescents, children ages 10-19, which make up approximately forty percent of the Afghanistan population, face significant struggles in their sexual reproductive health needs and elevated levels of reproductive health inequality, contributing to the high maternal death ratio of 638 deaths per 10,000 births, making Afghanistan the country with the tenth worst maternal mortality rate [26]. This lack of health care for adolescent girls is paired with Afghanistan’s fertility rate of 58 births per 1,000 girls between the ages of 15 and 19 [2].
When young girls enter labor, they are at the highest risk of death and other health complications. Obstetric fistula, for example, is a result of
prolonged unassisted labor that causes incontinence and community alienation and is a condition that 1 in 4 Afghan girls and women suffer from [41, 62]. The inadequate healthcare system is the primary reason conditions like fistulas have a higher prevalence in Afghanistan than in countries like the United States. Luckily, when the United States defeated the Taliban-led Afghan government in late 2001, international donor countries prioritized the weak healthcare system. Such efforts led to an impressive decline in maternal deaths, an increase in modern contraceptives and midwives, but some women and girls continue to struggle to access basic health information, contraceptives, pre- and post-natal care, and modern cancer and fertility treatment [7]. The care the girls and women receive is also often poor quality, and they must travel far distances to access mediocre care [2, 7]. Fortunately, organizations like UNICEF, which is training healthcare professionals in Afghanistan and sharing sexual health impositions in “youth-friendly, gender-sensitive services,” are combatting healthcare inequity and providing girls and women with the best available healthcare [26]. Another health risk in Afghanistan comes from normalizing child marriages and closely-spaced pregnancies, which can cause death for the young girl and her baby [7, 8]. Child marriage is a union where one or more parties are under 18, and in 2021, approximately twenty-eight percent of girls and women aged 15-49 had been married before they turned 18 [1, 19, 22]. Child marriages often occur when lower-income families sell their daughters for significant funds to wealthy, much older husbands or as a way to settle rivalries between families [13]. The main reason for the popularity of child marriage in Afghanistan is extreme poverty and gender inequality or inferiority of girls, which are also the leading causes of period poverty, stigmas, and shaming [1]. “The traditional society in the country considers a girl who … [experiences] ‘menarche’ as being ready for marriage and child bearing,” a misconception fueled by a lack of sexual education and gender disparity, linking the horrors of child marriages to menstruation [54].
With an increase in child marriages in Afghanistan, a third of girls between the ages of 15-19 give birth each year, and the country has one of the highest infant mortality rates, with 45 deaths
per 1,000 births [13, 38]. Since two in three deliveries in Afghanistan happen at home without an attendant, pregnancies for these young girls are significantly more dangerous [23]. Child marriages and early pregnancies also lead to girls being removed from school, contributing to Afghanistan’s low literacy rate. Alternatively, the Taliban banning girls aged 12 and older from going to school increases child marriages. Another misconception in Afghan culture is that after each menstruation cycle, a girl supposedly loses an opportunity to get pregnant; thus, once a girl reaches menarche, she is often rushed into marriage [5]. Generally, Afghanistan’s poor healthcare system impacts child marriages, infant mortality, menstruation, and life expectancy.
Being a woman in Afghanistan is beyond difficult. From the Taliban’s intensely misogynistic views to women’s lack of freedom to leave home without male permission, Afghanistan consistently violates women’s and girls’ rights. Examples of Afghanistan’s women’s rights violations as of September of 2021 include forcing girls to wear hijabs, banning women from complaining about or suing “their husbands or the men of the family,” and banning girls in grades six and above from attending school [7]. The effects of women’s rights violations on Afghan women are exhibited in a 1998 JAMA research journal about “Women’s Health and Human Rights in Afghanistan.” The journal shared that 97 percent showed signs of depression, 68 percent expressed that they had tightly restricted social activities, and 71 percent sensed a decline in physical health [9, 44, 47].
In a July 2022 interview, Afghan-born journalist Rukhsar Azamee provided a personal view regarding the Taliban’s infringement on women’s rights. Azamee shared how devastated she was about the Taliban’s re-gain of control in Afghanistan, primarily because the work she and her colleagues completed to provide Afghan girls and women with a better life was being tarnished by the Taliban’s oppressive policies. A highlight of how Azamee helped trailblaze more
rights for women includes her bravery in being one of the first women to drive, ultimately leading to her being “[literally] thrown out of the country.” Azamee also presented her thoughts about why the Taliban is adamant about stifling young girls’ education, as she assumes that educated women with substantial careers hold more authority, threatening Afghanistan’s patriarchy.
Despite the desperate situation in Afghanistan, are international countries obligated to help Afghan citizens, especially women and girls? America, for example, has a history of not supporting international women’s rights, especially during President George W. Bush’s administration when he scaled back on “women’s rights at home and abroad through a slew of executive orders, judicial appointments, and administrative rules” [21]. More recently, the United States’ departure from Afghanistan was also an attack on Afghan women’s rights and a violation of their freedom and dignity. Despite warnings about the fragility of women’s rights if the United States’ troops were to leave Afghanistan, President Biden, nonetheless, made the final decision to evacuate [24]. In the July 2022 interview, Azamee highlighted that she believes international countries must be accountable; she stated, “if they say they want to protect girls’ rights to go to school…[international governments should] make sure they can go to school” [5]. Despite the world’s awareness of Afghanistan’s situation, Afghan people believe international attention has “horrifically moved away from Afghanistan” [5]. The situation of women’s rights in Afghanistan will not get better on its own. It will take assistance from other international powers to overthrow the Taliban’s rule and restore the rights of Afghan girls and women. Thus, it can be inferred that international powers must be involved in Afghanistan to protect women’s rights. For example, Afghanistan’s healthcare system initially relied on the help of Western countries, which funded ninety percent of Afghan health clinics [37]. This international support ended, however, because most of those countries are boycotting a Taliban-led country. In regards to the healthcare system, the lack of funds has prohibited ambulances from affording fuel, and thus, physicians advise many patients to take taxis, find other modes of transportation, or ultimately forgo treatment [28].
Without international aid, Afghan citizens’ access to adequate medical care will continue to worsen.
Afghanistan has many areas that need to be fixed to create a higher quality menstruation experience, and general livelihood, for girls and women. Nevertheless, before changes to how Afghans approach menstruation and female healthcare begins, Afghans must change how it approaches women’s rights. Presently, the legal age of consent is 16 years for females and 18 years for males [3]. However, if the legal age of consent for females were also raised to be 18, there would be a decrease in child pregnancies that result from child marriages and misogynistic beliefs. Such change would protect the lives of young Afghan girls and act as a precedent to further shifts to improve the lives of Afghan females.
For instance, another prominent issue in Afghanistan is a lack of affordable menstruation products and accessible places to acquire such products. However, if public venues like libraries and hospitals stocked themselves with menstruation products, impoverished girls and women could better access such products. Next, another problem that affects Afghan girls is the inappropriate toilet facilities in schools due to a lack of clean running water, custodial services, and separate-gendered bathrooms. To decrease the prevalence of this issue, the Afghanistan government will need to put more funding toward education. Afghanistan has total spending of approximately $11 billion, but only about $17 per capita is used for education [25]. If Afghanistan starts to spend more money on its education sector, schools will be able to afford more sanitation efforts and can work on improving access to clean water.
Lastly, Afghanistan needs more physicians, male and female, in rural areas. A 2018 World Health Organization study found that “Afghanistan has the second lowest health workforce density and the highest level of rural residing population in the Eastern Mediterranean Region” [1, 50]. In 2016, Afghanistan had a total of 0.3 physicians per 1,000 people and only 0.1 midwives and nurses per 1,000 people [40, 46]. Such alarmingly small numbers prove that Afghan citizens living in rural communities lack appropriate healthcare. Therefore, to
improve Afghanistan’s healthcare system in rural areas, female doctors should be legally permitted to work without male permission, and the government should increase its spending on healthcare. sMoreover, along with enhancing the healthcare system in rural areas, Afghanistan needs to improve girls’ and women’s ability to access medical care. An Afghan doctor working in Balkh, Afghanistan, realized quickly in his career that many of his patients walked seven hours to the closest medical facility; he also noticed that many female patients who had deteriorated health conditions often sought treatment “too late” because they needed permission and accompaniment from a maharam2 [17]. Since women are required to get male permission before going out, many women forgo getting medical assistance or receive care at home. So to relieve this terrible problem, women should be permitted to go out without permission if they seek medical care.
This paper reviewed poverty, stigmas associated with menstruation, and period shame affect Afghan girls. The prominent issue addressed in the paper was the evident lack of sex education in Afghanistan, resulting in over 50% of girls having limited knowledge about periods before menarche [52]. Since Afghan culture believes sex education is inappropriate, many misconceptions about menstruation have formed, like that washing oneself while menstruating can cause barrenness [59]. Another problem discussed in the paper is that the lack of toilet facilities in schools forces schoolgirls to miss school and eventually drop out, contributing to Afghanistan’s high illiteracy rates and high unemployment rates for women. “Period poverty,” due to the lack of accessible period products, especially in rural areas, is another problem addressed in the paper. Other issues addressed include the flawed healthcare system, its adverse effects on women’s ability to receive medical care, and the Taliban’s violation of women’s rights.
Afghan girls have limited access to pre-pubescent and menstrual education, menstrual hygiene management products, and effective pain relievers, putting them at greater risk of gynecological conditions and higher rates of maternal mortality.
I recommended there be more physicians in rural communities, women should be permitted to leave the house without male permission if they are doctors or are seeking medical care, menstrual hygiene products should be available in all public restrooms, and more government spending on the education sector and the age of consent for females should be raised to 18 years old, to improve the menstruation experience for Afghan girls and women. However, this is very difficult to enforce given the current political climate of Afghanistan, which is under control by the extremist Taliban group. The topics discussed in this paper are of great significance because 29.2% of Afghanistan’s population are illiterate females, and this number will continue to increase unless Afghanistan makes the recommended changes and international countries continue to support Afghan women’s rights to ensure girls feel comfortable going to school while menstruating [58].
1 The term was first used after many South Korean girls were found using shoe insoles as sanitary napkins because they could not afford actual pads. These girls inspired a Lunapads documentary and marked the beginning of a new age, where the South Korean government began actively combatting “period poverty.” 2A male companion, such as a husband or an in-law. Under the latest rules set by the Taliban, such male companions must accompany women whenever they travel outdoors.
[1] “About Child Marriage.” Girls Not Brides, www.girlsnotbrides.org/about-child-marriage/#sources. Accessed 5 July 2022.
[2] “Adolescent Fertility Rate (births per 1,000 Women Ages 15-19) - Afghanistan.” The World Bank, data.worldbank.org/ indicator/SP.ADO.TFRT?end=2020&locations=AF&most_recent_value_desc=false&start=1960&view=chart. Accessed 27 June 2022.
[3] “Afghanistan: National Child Protection Legislations.” International Centre for Missing and Exploited Children, July 2018, www.icmec.org/wp-content/uploads/2018/07/ICMEC-Afghanistan-National-Legislation-Updated.pdf. Accessed 22 July 2022.
[4] Anand, Tanu, and Suneela Garg. “Menstruation Related Myths in India: Strategies for Combating It.” Journal of Family Medicine and Primary Care, vol. 4, no. 2, 2015, p. 184, https://doi.org/10.4103%2F2249-4863.154627. Accessed 20 June 2022.
[5] Azamee, Rukhsar. Videoconference interview with the author. 13 July 2022.
[6] “Bacterial Vaginosis.” Office on Women’s Health, 31 May 2022, www.womenshealth.gov/a-z-topics/bacterial-vaginosis. Accessed 16 June 2022.
[7] Barr, Heather. “I Would like Four Kids — If We Stay Alive”: Women’s Access to Health Care in Afghanistan. Edited
by Dani Haas and Tom Porteous, 6 May 2021. Human Rights Watch, www.hrw.org/report/2021/05/06/i-would-four-kids-ifwe-stay-alive/womens-access-health-care-afghanistan. Accessed 28 June 2022.
[8] “List of Taliban Policies Violating Women’s Rights in Afghanistan.” Human Rights Watch, 29 Aug. 2021, www. hrw.org/news/2021/09/29/list-taliban-policies-violating-womens-rights-afghanistan. Accessed 18 July 2022.
[9] Biden, Joe. “Remarks by President Biden on the End of the War in Afghanistan.” 31 Aug. 2021. The White House, 31 Aug. 2021, www.whitehouse.gov/briefing-room/speeches-remarks/2021/08/31/remarks-by-president-biden-on-the-end-ofthe-war-in-afghanistan/. Accessed 22 June 2022. Address.
[10] Carroll, Linda. “Even in the U.S., Poor Women Often Can’t Afford Tampons, Pads.” Reuters, 10 Jan. 2019, www. reuters.com/article/us-health-menstruation-usa/even-inthe-u-s-poor-women-often-cant-afford-tampons-padsidUSKCN1P42TX. Accessed 21 June 2022.
[11] CDC. “CDC at Work: Global Water, Sanitation ad Hygiene (WASH).” Centers for Disease Control and Prevention, 3 Nov. 2021, www.cdc.gov/healthywater/global/programs/index. html. Accessed 22 June 2022.
[12] Chen, Jenny. “Afghan Women and Their Menstrual Dignity and Rights.” I Support the Girls, isupportthegirls. org/afghan-women-and-their-menstrual-dignity-and-rights/. Accessed 28 June 2022.
[13] “Child Marriage.” UNFPA Afghanistan, afghanistan.unfpa.org/en/node/15233. Accessed 4 July 2022.
[14] Chong, Linda. “Period Poverty in South Korea.” The Borgen Project, 6 Mar. 2021, borgenproject.org/period-poverty-in-south-korea/. Accessed 19 June 2022.
[15] Committee on the Elimination of Discrimination Against oen. “Committee on the Elimination of Discrimination against Women Opens Its Eighty-Second Session.” United Nations: Human Rights Office of the High Commissioner, 13 June 2022, www.ohchr.org/en/press-releases/2022/06/ committee-elimination-discrimination-against-women-opens-its-eighty-second. Accessed 22 June 2022.
[16] Deck, Jerica. “Seoul Offers Free Menstrual Products to Help End Period Poverty.” Global Citizen, 12 Oct. 2018, www. globalcitizen.org/en/content/south-korea-period-products/. Accessed 20 June 2022.
[17] “A Doctor’s View of Afghanistan’s Rural Healthcare.” World Bank Blogs, 17 Sept. 2019, blogs.worldbank.org/endpovertyinsouthasia/bringing-health-care-community-local-issues-are-best-solved-local-solutions. Accessed 22 July 2022.
[18] “Douching.” Office on Women’s Health, 22 Feb. 2021, www.womenshealth.gov/a-z-topics/douching. Accessed 16 June 2022.
[19] Efevbera, Yvette, and Jacqueline Bhabha. “Defining and Deconstructing Girl Child Marriage and Applications to Global Public Health.” BMC Public Health, vol. 20, no. 1, 15 Oct. 2020, https://doi.org/10.1186/s12889-020-09545-0. Accessed 4 July 2022.
[20] Fetrat, Sahar. “Afghanistan – Menstruation Challenges for Afghan Girls.” Women’s UN Report Network, 14 July 2016, wunrn.com/2016/07/afghanistan-menstruation-challenges-for-afghan-girls/. Accessed 26 June 2022.
[21] Filipovic, Jill. “America Has Abandoned the Women of Afghanistan.” CNN, 18 Aug. 2021, www.cnn.com/2021/08/18/ opinions/america-abandoning-afghanistan-women-filipovic/ index.html. Accessed 12 July 2022.
[22] Fore, Henrietta. “Girls Increasingly at Risk of Child Marriage in Afghanistan.” UNICEF, 12 Nov. 2021, www.unicef.org/ press-releases/girls-increasingly-risk-child-marriage-afghanistan. Accessed 4 July 2022.
[23] Fotheringham, Claire. “Reducing Risks for Pregnant Women.” Médecins Sans Frontières, 6 Mar. 2017, www.msf. org/afghanistan-reducing-risks-pregnant-women. Accessed 5 July 2022.
[24] Gibbons-Neff, Thomas, et al. “Afghan Women Fear the Worst, Whether War or Peace Lies Ahead.” New York Times,
18 Apr. 2021, www.nytimes.com/2021/04/18/world/asia/women-afghanistan-withdrawal-us.html. Accessed 18 July 2022.
[25] Haque, Tobias. “Where Does the Money Go? Examining Public Spending in Afghanistan.” World Bank Blogs, 29 July 2019, blogs.worldbank.org/endpovertyinsouthasia/ where-does-money-go-examining-public-spending-afghanistan. Accessed 22 July 2022.
[26] “Health: Unicef Afghanistan.” Unicef, www.unicef.org/ afghanistan/health. Accessed 27 June 2022.
[27] Julia Johns, et al. “Around the World in 28 Periods.” Women’s Health Magazine, 27 May 2016, www.womenshealthmag.com/life/a19974024/periods-around-the-world/. Accessed 14 June 2022.
[28] Jung, Elaine, and Hafizullah Maroof. “Giving Birth under the Taliban.” BBC News, 20 Sept. 2021, www.bbc.com/news/ world-asia-58585323. Accessed 5 July 2022.
[29] Landis, Debbie, et al. Girl-Driven Change Meeting the Needs of Adolescent Girls during COVID-19 and beyond. Compiled by CARE, Oct. 2020. CARE, www.care.org/wp-content/uploads/2020/10/CARE-USA-Adolescent-Girls-andCOVID-19-FINAL-Report.pdf. Accessed 22 June 2022.
[30] “Life Expectancy at Birth, Female (years) - Afghanistan.”
The World Bank, 2019, data.worldbank.org/indicator/SP. DYN.LE00.FE.IN?end=2020&locations=AF&most_recent_value_desc=true&start=1960&view=chart. Accessed 27 June 2022.
[31] “Life Expectancy at Birth, Female (years) - Iran, Islamic Rep.” The World Bank, 2019, data.worldbank.org/indicator/ SP.DYN.LE00.FE.IN?end=2020&locations=IR&most_recent_value_desc=true&start=1960&view=chart. Accessed 2 July 2022.
[32] “Life Expectancy at Birth, Female (years) - United States.” The World Bank, 2019, data.worldbank.org/indicator/SP. DYN.LE00.FE.IN?end=2020&locations=US&most_recent_value_desc=true&start=1960&view=chart. Accessed 2 July 2022.
[33] Majumdar, Samirah. “5 Facts about Religion in India.” Pew Research Center, 29 June 2018, www.pewresearch.org/ fact-tank/2018/06/29/5-facts-about-religion-in-india/. Accessed 20 June 2022.
[34] McCartney, Julia. “Further Improvements to Women’s Healthcare in Afghanistan.” Borgen Project, 19 Feb. 2018, borgenproject.org/womens-healthcare-in-afghanistan/. Accessed 27 June 2022.
[35] McConnell, Jamie. “Sixty-Two Menstrual Equity Laws Passed in the United States.” Women’s Voices for the Earth, 2 May 2022, www.womensvoices.org/2022/05/02/sixty-two-menstrual-equity-laws-passed-in-the-united-states/. Accessed 21 June 2022.
[36] “Menstrual Leave: South Korea Airline Ex-CEO Fined for Refusing Time off.” BBC News, 25 Apr. 2021, www.bbc.com/ news/world-asia-56877634. Accessed 20 June 2022.
[37] Moreno, Laura Bornstein. “Women’s Healthcare in Danger under Taliban Rule.” Human Rights Pulse, 29 Nov. 2021, www.humanrightspulse.com/mastercontentblog/womens-healthcare-in-danger-under-taliban-rule. Accessed 28 June 2022.
[38] “Mortality Rate, Infant (per 1,000 Live Births).” The World Bank, 2020, data.worldbank.org/indicator/SP.DYN.IMRT. IN?most_recent_value_desc=true&view=map. Accessed 4 July 2022.
[39] Nader, Zahra. “’We Have to Fight Back.’ Afghan Women Are Losing Their Hard-Won Right to Work under the Taliban.” Time, 17 May 2022, time.com/6177133/afghan-women-workforce-challenges-taliban/. Accessed 21 July 2022.
[40] “Nurses and Midwives (per 1,000 People) - Afghanistan.” The World Bank, 2018, data.worldbank.org/indicator/ SH.MED.NUMW.P3?locations=AF&start=2006. Accessed 22 July 2022.
[41] “Obstetric Fistula.” UNFPA Afghanistan, afghanistan. unfpa.org/en/node/15226. Accessed 4 July 2022.
[42] Odey, Goodness Ogeyi, et al. “Knowledge and Practice of Menstrual Hygiene among Adolescent Girls in Secondary
Schools of Herat, Afghanistan.” Razi International Medical Journal, vol. 2, no. 1, 24 May 2022, pp. 1-9, https://doi. org/10.56101/rimj.v2i1.20. Accessed 15 June 2022.
[43] O’Donnell, Lynne. “The Taliban Have Made the Burqa Mandatory Again.” Foreign Policy, 9 May 2022, foreignpolicy. com/2022/05/09/taliban-women-burqa-afghanistan-control/. Accessed 21 July 2022.
[44] Patton-Bey, Suad. “A Lot of Muslim Women Don’t Use Tampons, but It’s Not for the Reasons You Think.” The Tempest, 5 Sept. 2017, thetempest.co/2017/09/05/life-love/muslim-women-tampons/. Accessed 19 June 2022.
[45] Period. End of Sentence. Directed by Rayka Zehtabchi, produced by Melissa Berton, Netflix, 2018.
[46] “Physicians (per 1,000 People) - Afghanistan.” The World Bank, 2016, data.worldbank.org/indicator/SH.MED.PHYS. ZS?locations=AF&start=2006. Accessed 22 July 2022.
[47] Rasekh, Zohra, et al. “Women’s Health and Human Rights in Afghanistan.” JAMA, vol. 280, no. 5, 5 Aug. 1998, p. 449, https://doi.org/10.1001/jama.280.5.449. Accessed 18 July 2022.
[48] Read, Claire. “The Country Where Tampons May Cause a Security Alert.” BBC News, 12 June 2017, www.bbc.com/news/ magazine-40221010. Accessed 19 June 2022.
[49] Safepad™ Is a Reusable Sanitary Pad. Real Relief, www. realreliefway.com/products/safepad. Accessed 22 June 2022.
[50] Safi, Najibullah, et al. “Addressing Health Workforce Shortages and Maldistribution in Afghanistan.” Eastern Mediterranean Health Journal, vol. 24, no. 09, 1 Sept. 2018, pp. 951-58, https://doi.org/10.26719/2018.24.9.951. Accessed 22 July 2022. Abstract.
[51] Shakib, Mohammad Kazem. “Afghan University Women Graduates Are Not Well-Represented in the Job Market in Afghanistan.” Master’s Theses, 2014, pp. x-3, ecommons.luc. edu/luc_theses/2243. Accessed 21 July 2022.
[52] Sherzai, Ajmal. “Breaking Taboos.” Unicef Afghanistan, 27 May 2021, www.unicef.org/afghanistan/stories/breaking-taboos. Accessed 15 June 2022.
[53] Smith, Amy. “The State of Period Poverty in the U.S.” Penn Nursing, 2019, www.nursing.upenn.edu/details/news. php?id=1545. Accessed 21 June 2022.
[54] Stankezai, Zahida. “It Was Almost Surreal.” UNICEF Afghanistan, 21 Nov. 2018, www.unicef.org/afghanistan/stories/ it-was-almost-surreal. Accessed 5 July 2022.
[55] Stubbs, Margaret L. “Cultural Perceptions and Practices around Menarche and Adolescent Menstruation in the United States.” Annals of the New York Academy of Sciences, vol. 1135, no. 1, June 2008, pp. 58-66, https://doi.org/10.1196/annals.1429.008. Accessed 21 June 2022. Abstract.
[56] “Teacher Average Salary in Afghanistan 2022.” Salary Explorer, 2022, www.salaryexplorer.com/salary-survey. php?loc=1&loctype=1&job=123&jobtype=3. Accessed 9 July 2022.
[57] “The 10 Worst Places to Be a Mother.” Concern Worldwide US - Humanitarian Organization, 4 May 2022, www.concernusa.org/story/worst-countries-to-be-a-mother/. Accessed 2 July 2022.
[58] “UNESCO Stands with All Afghans to Ensure Youth and Adults in Afghanistan, Especially Women and Girls, Achieve Literacy and Numeracy by 2030.” [59] UNESCO, 9 Aug. 2021, en.unesco.org/news/unesco-stands-all-afghans-ensure-youth-and-adults-afghanistan-especially-women-and-girls. Accessed 16 June 2022.
[60] United Nations Fund for Population Activities. “Menstruation and Human Rights - Frequently Asked Questions.” United Nations Population Fund, May 2022, www.unfpa.org/ menstruationfaq. Accessed 15 June 2022.
[61] Watson, Grace. “Period Poverty in Afghanistan.” The Borgen Project, 11 July 2021, borgenproject.org/period-poverty-in-afghanistan/. Accessed 16 June 2022.
“Period Poverty in Afghanistan after the US Withdrawal.” Borgen Magazine, 5 Jan. 2022, www.borgenmagazine.com/period-poverty-in-afghanistan/. Accessed 16 June 2022.
[62] “What Is Fistula?” Fistula Foundation, fistulafoundation
In static advertisements, images instead of videos are used to promote products, and it is most often the faces of the models that get the consumers’ attention. How facial expressions transfer emotions from the producer to the consumer has been explained by the emotional contagion theory, which suggests the recipient replicates the emitter through motor mimicry which changes the recipient’s emotional state. This paper investigates the effects of facial expressions in advertisements on adolescents in comparison to adults. It was hypothesized that participants would give a higher product evaluation if the model displayed a real smile, versus a fake smile or neutral facial expression. It was also hypothesized that adolescents would be more susceptible to facial expressions and therefore pay more for the same product than adults. High school and adult participants completed a survey in which they evaluated a product (a white t-shirt) when the model displayed a neutral face, fake smile, and real smile. The results showed that participants gave higher product evaluation scores and were willing to pay more when the model displayed a genuine smile over the other facial expressions. In addition, adolescents were less influenced by the model’s expressions than adults which may be due to their lack of experience in spending. To further this study, the effects of emotional contagion on other factors including how the product attracts attention or lasts in the consumers’ memory could be tested.
Every day, American consumers spend $29.3 billion on average.¹ Annually, $296.4 billion US dollars are spent on advertising for these products.² Human faces are oftentimes at the center of these marketing campaigns. Adolescents are one of the key targets of advertising perhaps due to their susceptibility to influence.³ This paper investigates the effects of facial expressions in advertisements on adolescents. For the purpose of this paper, we will adopt the following definitions. First, facial expressions display a person’s discrete emotional state. Second, the effect of advertisements can be assessed through the willingness to purchase. The willingness to purchase, as defined in behavioral economics, is the highest price a consumer is willing to pay for a product. Third, adolescents are defined biologically as individuals in the 10-19 years age group in a transitional phase of growth and maturation through puberty to adulthood. Adolescence marks a time of substantial change in physical maturation, cognitive abilities, and social interactions.
In static advertisements, images, instead of videos, are used to promote the product. A variety of facial expressions and postures are emphasized by models in order to convey the appropriate message. It is most often the faces of the models that get the consumers’ attention.4 How facial expressions transfer emotions from the producer to the consumer has been explained by the emotional contagion theory.5 This theory has two steps: at first, the recipient replicates the emitter through motor mimicry. Second, mimicry changes the recipient’s emotional state. While different types of smiles have been studied, this paper investigates the effects of a fake smile compared to a genuine smile and a neutral facial expression on product evaluation.
This paper expands on recent findings by Isabella and Vieira who investigated emotional contagion effects in advertisements.6 This denotes the process in which emotions and related behavior are spread from sender to receiver. One of their experiments tested the influence of neutral, fake, and genuine smiles of a model on the product evaluation. The experiments did in fact prove that the facial expressions influenced the product evaluation. They showed that genuine smiles tend to increase product evaluation over
false smiles because of the mimicry response.
The brain is not fully developed until 25. Pechmann studies how adolescents are more impulsive and self-conscious than adults and are therefore more susceptible to fall for advertisements.³ Especially with the growing prevalence of social media and technology on youth, it is important to see how the behavior of the new generation is affected, if at all, by facial expressions. Based on the previous research of facial expressions on advertisements outlined above, the hypotheses for the present study are the following:
Hypothesis 1: All participants will be most susceptible to a genuine smile over a fake smile and a fake smile over a neutral facial expression.
Hypothesis 2: Adolescents will be more susceptible to advertisements and therefore will be more willing to pay a higher price for the same product compared to adults.
Hypothesis 3: Adolescents will be more susceptible to facial expressions and therefore a genuine smile will produce an even more positive influence in product evaluation for adolescents than adults.
In this study, the participants were separated into two groups: adults [n = 30] and adolescents [n = 20]. The survey was administered to an online panel of adults older than 19 in the Fall of 2021. 99 responses were received, and 30 were randomly selected for the analysis so that the sample of children and adults had balanced numbers. The same survey was distributed at a high school in New Jersey. The procedure of the present study replicated that of Isabella and Viera.6 A survey was conducted that showed pictures of a female face with a neutral expression, fake smiling, and genuinely smiling in a static advertisement. In this survey, the product being sold was a plain white t-shirt. Before measuring the willingness to purchase, a control measure was included in the survey to check for congruence between the model’s facial expression and the participant’s interpretation of it. This control was used to eliminate participants. 29 adults and 3 adolescents were not included in the results because they did not accurately perceive the model’s facial expression. Participants then rated their appeal and judg-
ment of the product based on questions from the Beren’s Product evaluation scale, which asks participants to rate the favorability, their likelihood of purchasing the product, and the preferred pricing of the product on a 7-point Likert scale.7
In total, the survey responses from 30 adult participants were randomly selected and analyzed. Of these, 26.67% were female and 73.33% were male. 73.33% of participants identified as Caucasian, 10% identified as African American, and 23.33% identified as Asian American or Pacific Islander.
In total, 20 adolescent participants answered the survey. Of these, 75% were female, 20% were male, and 5% preferred not to disclose their gender. 50% of participants identified as Caucasian, 5% identified as African American, 45% identified as Asian American or Pacific Islander, and 5% identified as Hispanic. The survey was an online survey sent to a high school and participants were included in an optional raffle as an incentive for participation.
The two main aspects of the survey that were analyzed were the adult and adolescent participants’ price evaluation and judgment of the quality of the product.

Single factor ANOVAs were conducted to compare the responses of both adults and adolescents for both quality and preferred pricing across the three faces: neutral, fake smile, real smile. On average, there was a statistically significant difference in adults’ rating of product quality across a neutral face (M = 2.53, SD = 1.48), fake smile (M = 4.37, SD = 1.47), and real smile (M = 4.2, SD = 2.02); F(2, 87) = 10.94, p < 0.0001. (Figure 1.1) In addition, there was a statistically significant difference in adults’ ratings for preferred pricing across a neutral face (M = 6.67, SD = 5.55), fake smile (M = 9.6, SD = 6.67), and real smile (M = 11.13, SD = 8.61); F(2, 87) = 3.1, p < 0.05. (Figure 1.2) On average, there was a statistically significant difference in adolescents’ ratings of product quality across the neutral face (M = 2.55, SD = 1.50), fake smile (M = 3.8, SD = 1.48), and real smile (M = 3.9, SD = 2.02) conditions; F(2, 57) = 3.98, p < 0.025. (Figure 1.3) Lastly, there was a statistically significant difference in
adolescents’ ratings for preferred pricing across a neutral face (M = 6.92, SD = 2.89), fake smile (M = 9.32, SD = 3.91), and real smile (M = 10.17, SD = 3.96); F(2, 57) = 4.33, p < 0.018. (Figure 1.4)



Figure 1A. Average quality rating on Beren’s scale of adults across three facial expressions
Figure 1B. Average product pricing for adults across three facial expressions
Figure 1C. Average quality rating on Beren’s scale for adolescents across three facial expressions
Figure 1D. Average product pricing for adolescents across three facial expressions
Adults: To further explore the results from the ANOVA analysis, paired sample t-tests were used to determine which facial expression conditions were in fact statistically different. According to the results of the Paired Sample T-Test, adults’ ratings of quality across a neutral face and fake smile (p < 0.00001) as well as across a neutral face and real smile (p < 0.001) were both statistically significant. On the other hand, adults’ ratings across a fake smile and real smile were not statistically significant (p = 0.54). The adults’ ratings of product pricing across a neutral face and fake smile (p < 0.002), neutral face and real smile (p < 0.003), and fake smile and real smile (p < 0.03) were all statistically significant.
Adolescents: According to the results of the Paired Sample T-Test, adolescents’ ratings of quality across a neutral face and fake smile (p < 0.006) as well as across a neutral face and real smile (p < 0.02) were both statistically significant. On the other hand, adolescents’ ratings across a fake smile and real smile were not statistically significant (p > 0.05). Adolescents’ ratings of product pricing across a neutral face and fake smile (p < 0.0002), neutral face and real smile (p < 0.0002), and fake smile and real smile (p < 0.04) were all statistically significant.
These findings support previous research on emotional contagion in which happiness exemplified by the advertiser evokes that same emotion in the participants. In all cases, adolescents and adults were on average willing to pay more and had a significantly higher evaluation of the quality of the product when the advertiser was smiling rather than displaying a neutral facial expression.
The results show that participants placed higher evaluation scores when presented with an advertiser with a genuine smile. To explain these results, we turn to previous studies, which have revealed that the muscular contractions involved in smiling and fake smiling can actually underlie how a viewer regards the expression and how positive emotions are transferred.8-9 With a genuine smile, the zygomatic major and orbicularis oculi muscles in the face are contracted. As a consequence, people tend to intuitively evaluate a smiling facial expression as authentic, genu-
ine, and trustworthy.¹º In the present study, it is possible that the advertisers’ genuine smile inspired a sense of trust and positivity in the participants viewing the product, leading to higher product quality ratings in the smiling condition. On the other hand, a fake smile only involves the zygomatic major muscle.¹¹ The data shows that consumers can subconsciously observe the difference between a genuine and false smile and be affected differently. In the present study, it appears that participants differentially responded to a genuine versus fake smile as their product quality ratings and price ratings differed.
It was hypothesized that adolescents would pay a greater amount than adults for the same product due to their lack of experience earning and spending money. The results of this study surprisingly showed that the adolescents overall had a lower evaluation of the product. For both product quality and preferred pricing for all three facial expressions, adolescents, on average, gave a lower rating or price for each category compared to adults. For adolescents, the greater the susceptibility to interpersonal influence, the greater the tendency to buy on impulse.¹² Therefore, when shown a model with a genuine smile, adolescents were more willing to pay a higher price and give the product a better evaluation, which is also demonstrated by the results. However, adolescents were significantly less influenced by a false versus a real smile when pricing the product compared to adults. On average, adults were willing to pay about 14.6% more when the model had a real smile while adolescents were willing to pay about 9.12% more. This can be attributed to the lack of exposure of adolescents to spending, which results in an unformed understanding of the value of money. Part of this study involved having participants imagine that this was a real product being sold. In other words, they had to envision that this still face on the screen was associated with selling this white t-shirt. Perhaps adults were better able to envision this scenario, while adolescents struggled to make this link.
In conclusion, consumers are willing to pay more and tend to have a higher evaluation of the quality of a product when a model in an advertisement displays a real smile, instead of a fake
smile or a neutral facial expression in marketing material. Adults have a better evaluation and are willing to pay more than adolescents for the same product. Lastly, adults are more susceptible to facial expressions since their price evaluation when presented with a fake smile to a real smile changed more dramatically than that of adolescents. These implications suggest marketing managers would benefit from advertising with genuine smiles since they will evoke positive emotions in the consumer via emotional contagion, making them more likely to purchase.
The study could have benefitted from a larger sample size to increase the amount of data collected. This would improve the validity and generalizability of the data. Future investigations could explore the effects of emotional contagion on other factors including how the product attracts attention or lasts in the consumers’ memory. Different stimuli such as the race or gender of the advertiser instead of facial expression could be used to measure product evaluation. This study researched the effects of facial expressions in static advertisements, and future research could also replicate this with dynamic advertisements (i.e., moving images for instance).
Thank you Florentine Salmony and Hannah Dunn for mentoring and helping me with my project.
[1] Brittany De Lea. (2019, March 12). How much the average American spends per day. Fox Business; Fox Business https://www.foxbusiness.com/personal-finance/how-much-the-average-american-spends-perday
[2] North America ad spend 2024 | Statista. (2021). Statista; Statista. https://www.statista.com/statistics/429036/advertising-expenditure-in-north-america/
[3] Pechmann C, Levine L, Loughlin S, Leslie F. Impulsive and Self-Conscious: Adolescents’ Vulnerability to Advertising and Promotion. Journal of Public Policy & Marketing. 2005;24(2):202-221. https://doi. org/10.1509/jppm.2005.24.2.202
[4] Bindemann, M., Burton, A. M., Langton, S. R. H., Schweinberger, S. R., & Doherty, M. J. (2007). The control of attention to faces. Journal of Vision, 715,
1–8. https://doi.org/10.1167/7.10.15
[5] Dallimore, K. S., Sparks, B. A., & Butcher, K. (2007). The influence of angry customer outbursts on service providers’ facial displays and affective states. Journal of Service Research, 10, 78–92. https://doi. org/10.1177/1094670507304694
[6] Isabella, G., & Vieira, V.A. (2020). The effect of facial expression on emotional contagion and product evaluation in print advertising. RAUSP Management Journal, 55, 375-391. https://doi.org/10.1108/ RAUSP-03-2019-0038
[7] Berens, G., Van Riel, C. B., & Van Bruggen, G. H. (2005). Corporate associations and consumer product responses: The moderating role of corporate brand dominance. Journal of Marketing, 69, 35–48.https:// doi.org/10.1509/jmkg.69.3.35.66357
[8] Hennig-Thurau, T., Groth, M., Paul, M., & Gremler, D. D. (2006). Are all smiles created equal? How emotional contagion and emotional labor affect service relationships. Journal of Marketing, 70, 58–73. https://doi.org/10.1509/jmkg.70.3.58
[9] Howard, D. J., & Gengler, C. (2001). Emotional contagion effects on product attitudes. Journal of Consumer Research, 28, 189–201. https://doi. org/10.1086/322897
[10] Gunnery, S. D., & Ruben, M. A. (2015). Perceptions of duchenne and non-duchenne smiles: A metaanalysis. Cognition and Emotion, 9931, 1–15. https://doi.org/10.1080/02699931.2015.1018817
[11] Messinger, D. S., Mattson, W. I., Mahoor, M. H., & Cohn, J. F. (2012). The eyes have it: Making positive expressions more positive and negative expressions more negative. Emotion, 12, 430–436.https://doi. org/10.1037/a0026498
[12] Lin, Yi-Hsiu, and Chen-Yueh Chen. “Adolescents’ impulse buying: susceptibility to interpersonal influence and fear of negative evaluation.” Social Behavior and Personality: An International Journal, vol. 40, no. 3, Apr. 2012, pp. 353+. https://doi.org/10.2224/ sbp.2012.40.3.353
A study conducted by 29 scientists has uncovered the nature of an exercise-inducible metabolite that can help suppress feeding and obesity. The study concentrates on the metabolite N-lactoyl-phenylalanine (Lac-Phe), a signaling metabolite in the bloodstream that can offer protection against obesity. Although the study was conducted on mice, the presence of this metabolite has been found in a variety of mammals, from racehorses to humans.
One of the fascinating functions of the LacPhe metabolite is its ability to improve glucose homeostasis, which can help prevent type 2 diabetes. For instance, in obese mice, mediated increases in Lac-Phe reduce food intake while having no affect on energy or movement. During the experiment, mice were first run on a treadmill until exhaustion, to simulate exercise. Then, the scientist performed both targeted and untargeted metabolomics (the study of metabolites within an organism) on the blood plasma of mice in order to collect the data in an unbiased manner.
While the scientists noticed an increase in metabolites like lactate, they were surprised to see that the most significant change was found by the untargeted metabolomics. The chemical formula and mass to charge ratio only confirmed this, as it didn’t match any metabolite in their original list. This metabolite was assigned to a conjugate of lactate and phenylalanine also known as Lac-Phe. Plasma Lac-Phe levels in mice dramatically in-
creased after their run and took 1 hour to return back to base levels. The scientists were able to conclude that the Lac-Phe metabolite is able to circulate best during exercise. However, scientists still needed to observe how the production of this metabolite suppresses obesity. After noticing that Lac-Phe is produced in Cnpd2 cells, the scientists used mice that had their Cndp2 cells removed. The two groups in this part of the experiment were the Wild Type mice (WT), and the CNDP2-KO which had no Cnpd2 cells. It was shown that after 40 days, the CNDP2-KO mice were on average 7 grams heavier, with most of the additional weight being in the tissue. Additionally, during exercise, WT mice had 3 times the Lac-Phe concentration when compared to the CNDP2KO, while the CNDP2-KO had a greater cumulative food intake. Therefore, Cnpd2 cells are the primary producers of the Lac-Phe metabolite and are equally as vital in suppressing obesity.
In summation, this research proves that LacPhe, a metabolite in the bloodstream, is able to offer improved protection against obesity. Lac-Phe also allows for the subject to consume half as much food as it normally would after twelve hours. And without the Cnpd2 cell, there is a noticeable decrease in Lac-Phe production while tissue weight and food intake increase. The future of helping those who suffer from cardiometabolic diseases like obesity and type 2 diabetes may very well be down this avenue.
Chronic pain is a common condition that involves hypersensitivity of neurons. Many treatments currently target the neurons of the nervous system but often result in adequate responses. Located in the peripheral nervous system, satellite glial cells (SGCs) are a type of glial cell that closely envelope the cell bodies of neurons in the sensory ganglia. SGCs have been recently proven to play a direct role in the pathogenesis of pain. Because of their location early in the pain sensation pathway and their close relationship with neurons, they are a possible therapeutic target for chronic pain. The activation cascade of SGCs includes ATP, hypersensitivity of purinergic receptors, release of substance P and cytokine, changes in expression of ion channels, increased gap junction coupling, and a sustained calcium wave. There is evidence that these changes caused by SGCs contribute to chronic pain by altering neuronal activity. In particular, purinergic receptor P2X7R plays a major role in this cascade, promoting further ATP release, increasing ATP sensitivity, and contributing to a pro-inflammatory feedback loop. In this paper, we propose an experiment to block P2X7R with A-740003 as a treatment for chronic pain. Two tests would be conducted on rats. The first test would confirm P2X7R activation, A-740003 blockage, and downstream effects in vitro. The second test would determine the effect of blockage of P2X7R with A-740003 in animals exhibiting hyperalgesia in vivo. Hypothetical results suggest confirmation of the significant role of P2X7R in SGC activation, that A-740003 inhibits this role, and that A-740003 successfully returned animals with hyperalgesia to normal levels of nociception.

Pain perception, nociception, is the process by which noxious stimuli is relayed from the site of stimulation to the central nervous system (CNS), which includes the brain and spinal cord. Different from normal perception where somatic receptors are activated in response to non-painful stimuli, nociception involves different neural pathways (Freudenrich, 2007). Neurons called nociceptors are located in the peripheral nervous system (PNS) and are activated in response to painful stimuli (Freudenrich, 2007). Generally, there are three steps in the pain process: reception of stimuli, transmission, and pain center reception (Yam et al., 2018). Nociceptors are activated after sensing the stimulus. Nociceptors then transmit the pain signal from the PNS to the CNS. Finally, the brain processes the information and responds accordingly.

This paper focuses on a novel perspective on treating pathogenesis of the pain sensation pathway, specifically chronic pain, by focusing on the PNS. Sensory ganglia are clusters of nerve cell bodies located in the PNS that have a bifurcating axon. One end goes to the periphery, and the other goes to the spinal cord. The three main types of sensory ganglia include the trigeminal ganglia (TG), which innervates the head, face, and teeth; the nodose ganglia (NG), which innervates most of the visceral organs; and the dorsal root ganglia (DRG), which innervates the rest of the body (Hanani, 2015).
Shown in Fig. 1 is the innervation of the GI tract, involving the NG and the DRG; one end of each ganglion goes toward the periphery (i.e. the GI tract) and the other goes toward the spinal cord. Chronic pain usually originates from misfiring nociceptors in sensory ganglia, making it a viable target for pain treatment.
Pain serves a protective role as the warning system for the body to react to any possible threats, but it can also develop into
pathological pain which is nonprotective and maladaptive (“Classification of Chronic Pain”). Chronic pain is long-lasting pain that is usually caused by an initial injury but continues even after the underlying injury is resolved. Examples of chronic pain include arthritis (joint pain), back pain, neck pain, migraines, etc. As a result, chronic pain interferes with daily activities, affecting mobility and decreasing the general quality of life. It can become so debilitating that psychological problems like depression could arise.
Figure 1. A diagram (Hanani, 2015) showing the sensory innervation of the GI tract
Chronic pain is believed to occur after the nerves become damaged, triggering a process called central sensitization where nociceptors become hypersensitive to stimuli, firing off when they are not supposed to (“Classification of Chronic Pain”). Chronic pain can be classified as nociceptive or neuropathic (“Different Types of Chronic Pain,” 2020). While the differences are not important in this paper, their differentiation lies in the origin of the injury. Nociceptive pain develops in response to a specific stimulus to the body (e.g. fractures) that ultimately leads to long-term changes in the nervous system. Neuropathic pain occurs when the nervous system directly sustains damage, resulting in long-term
malfunctions in the nociceptors. Both types of chronic pain occur after central sensitization. Chronic pain therapies are complicated and often result in inadequate responses or side effects, so it is essential to improve our understanding of the mechanisms and cells involved in the generation and maintenance of chronic pain (Gazerani, 2021).
Pain therapies historically target the neurons themselves that transmit pain signals (Jasmin et al., 2010). However, there is increasing attention on the role of glial cells in pain modulation. Glial cells are part of both the CNS and PNS but do not directly participate in electrical signaling, a role associated specifically with neurons. Rather, they provide a myriad of supportive functions including modulation of the rate of nerve signal propagation, control of neural response to neurotransmitters, and structure for parts of neural development (Purves et al., 2001)). Recently, central glia, especially astrocytes and microglia, have also been identified to participate in the response to neural injury. Even though PNS is where pathogenesis of pain sensation originates, the roles of peripheral glia in pain modulation are significantly less researched and understood (Gazerani, 2021).
However, recent evidence shows that activation of satellite glial cells (SGCs) contributes to chronic pain through augmentation of neural activity (Hanani & Spray, 2020). SGCs are poorly understood glia that are exclusively located in the PNS where they envelope ganglionic neurons, including neurons of the DRG, TG, and NG. As a result, their processes are closely tied to the neuron they surround. Considering SGCs are located earlier in the pain pathway than central glial cells and close relationship with sensory ganglia, understanding and targeting their role in pain has the potential for decreasing the likelihood of pain chronification and for hindering the transition from peripheral to central sensitization. Therefore, SGCs and their mechanism in the pain pathway are a
promising field of study for pain research and can be a valuable target for therapeutic drugs.

SGCs are mononuclear cells found in the peripheral nervous system. Morphologically, SGCs are similar to Schwann cells in that they wrap around the neuronal cell body, commonly forming a sheath. Typically, a single sheath is made up of multiple SGCs connected together as seen in Fig. 2 and the top diagram of Fig. 3 (Hanani 2020). In neuronal groupings, cell bodies and their sheaths are commonly barely separated by connective tissue space. A small proportion of DRG neurons have been found to share an SGC envelope, forming a cluster with multiple neurons. Neurons inside of a cluster either have a single SGC separating them or have a thin layer of extracellular space as seen in the bottom two diagrams of Fig. 3 (Hanani 2020). SGCs typically maintain a 20 nm gap between themselves and the neuron which often projects into the SGC sheath (Hanani 2020). This closely tied structure allows for bidirectional and complex neuron-SGC and SGC-SGC interactions.
Figure 2. Low-power electron microscope showing SGCs (blue) and neurons (labeled N1-N6). Ct stands for connective tissue, and v stands for blood vessels. (Hanani & Spray, 2020)
Figure 3. Diagram showing three different ways SGC-neuron clusters can form (Hanani & Spray, 2020)
SGCs are physiologically similar to the astrocytes of the CNS, carrying out functions such as ion concentration regulation in extracellular space and neurotransmitter recycling. To communicate with other SGCs, neurons, and other bodies in extracellular space, SGCs also express numerous receptors and signaling factors, some of which are believed to mediate the generation and maintenance of chronic pain. The most notable include ATP, P2 purinergic receptors, calcitonin gene-related peptide (CGRP), substance P, glutamate, and cytokines. As a result, neuronal activity in sensory ganglia depends to a large degree on neuron-glia interactions, so understanding these interactions is highly valuable in understanding the development of chronic pain.
Originally believed to mainly play a passive role in the transmission of information from the periphery to the CNS, there is increasing evidence that SGCs have a much more active role. Neurons in sensory ganglia not only lack synaptic contact with each other, but they are also separated from each other by SGCs and extracellular space, so there should be no interactions between the neurons (Shinder & Devor, 1994). On the other hand, neurotrans-
mitter receptors have been found to be present in the cell bodies of sensory ganglia neurons. A calcium imaging study on DRG neurons in live mice found that the excitation of one DRG neuron sometimes led to the excitation of neighboring neurons. Called cross-excitation, this effect was found to occur more often after nerve injury and was associated with a calcium-dependent release of chemicals and neurotransmitters that alter the physiological characteristics of the neuron, resulting in changes in the excitability of nociceptive neurons. The fact that SGCs completely surround the cell body suggests that SGCs mediate cross-excitation. This idea of enhanced direct neuron-SGC and indirect neuron-neuron communication following injury is consistent with a number of recent studies on rodent pain models. The current suspected participants in this paracrine signaling mechanism include ATP and hypersensitivity of purinergic receptors, release of substance P and cytokine, changes in expression of ion channels, increased gap junction coupling, and a sustained calcium wave.
ATP is the primary messenger between neurons and SGCs in the pain pathway through a process called purinergic transmission. Pannexins (Panx) are homologs of gap junction proteins and form membrane channels that allow ATP to be released into extracellular fluid (Hanani & Spray, 2020). P2 receptors, a type of purinergic receptor, are found on the surface of neurons and are activated by extracellular ATP. There are seven ligand-gated ionotropic receptors (P2X1R-P2X7R) and eight G protein-coupled receptors (P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R-P2Y14R) (Inoue et al., 2005). Pharmacological and molecular biological analysis has shown that these receptors play a dominant role in mediating nociceptive response to extracellular ATP in the CNS (“Purinergic Receptor”). This relationship between ATP and purinergic receptors remains true in SGC-neuron interaction in response to neuropathic pain. Both

SGCs and neurons can release ATP through Panx1 channels, and both cell types express P2 receptors (Costa & Neto, 2015). Extracellular ATP binds to and activates P2 receptors and is taken in by the cell via endocytosis. Ecto-ATPases then break down ATP to ADP and other purines. In the DRG, ecto-ATPases are found in SGCs but not in neurons, enabling SGCs to regulate the ATP level in sensory ganglia (Hanani & Spray, 2020). Once P2X receptors are activated by ATP, their ion channels open, ultimately leading to further ATP release. Thus, the sensitivity to and level of ATP plays a major role in the level of pain response of sensory ganglia.
In several pain models including the DRG of rats following spinal nerve ligation (Zhang et al., 2015), the TG of orofacial pain models (Hanstein et al., 2016), and NG systemic inflammation models (Feldman-Goriachnik et al., 2015), it has been found that Panx1 expression is upregulated during chronic pain. This increased Panx1 expression enables greater amounts of ATP to be released in response to injury to neurons, thus further activating P2 receptors in both the SGCs and neurons.
It has also been shown that the sensitivity of SGCs to ATP dramatically increases following injury. Several studies demonstrated that this increased sensitivity is attributed to a shift in the P2 receptor subtype population in SGCs in response to nerve damage or inflammation. One such study of TG pain models recorded an increase in the sensitivity of SGCs to ATP in the TG by 100-fold (Kushnir et al., 2011). It was found that this was caused by a shift in the P2 receptor population. In healthy mice, the response of SGCs to ATP was primarily mediated by P2Y receptors. In comparison, in mice with inflammation, response to ATP was mediated by P2XR. Though the exact subtype of P2XR was not determined, the candidates included P2X2R, P2X4R, and P2X5R, but not P2X7R. In a similar study on DRG SGCs, P2X7R was upregulated in patients with chronic pain (Blum et al., 2014). These studies demonstrate that the expression of P2 receptor subtypes in SGCs shifts in response to injury. The exact P2XR subtype that the population shifts towards var-
ies depending on the type of sensory ganglia. In the second study previously mentioned, the primary P2 receptor subtype appeared to shift toward P2X7R in the DRG. In contrast, in the first study, researchers determined that P2X7R did not become prominent in TG following injury.
Furthermore, the level of exportable ATP in both SGCs and neurons depends upon the concentration of intracellular Ca2+, as the metabolism of ATP requires Ca2+. Therefore it is not surprising that there is not only an increase in sensitivity to ATP following injury, but there is also an increase in intracellular Ca2+, necessary to keep up with the levels of ATP metabolism. Because of this, the level of Ca2+ can be used as a marker for the activation of P2R. This is especially true for the activation of P2X7R. Focusing specifically on P2X7R, the activation of P2X7R is associated with the maturation and release of interleukin-1β (IL-1β), a potent inflammatory cytokine whose downstream effects are known to lead to the generation and maintenance of pain (Chessell et al., 2005). In a study looking to define the molecular physiology of the P2 receptor subtypes, P2X7R was also found to become more sensitive to ATP in low extracellular Ca2+ concentrations (North, 2002). As the concentration of intracellular Ca2+ increases, the concentration of extracellular Ca2+ decreases, further increasing the sensitivity of P2X7R. These two characteristics place P2X7R as a major target for chronic pain drugs. Ultimately, an excessive response to injury by P2X7R can lead to chronic pain by releasing large amounts of ATP, contributing to sustained P2X7R activation and a pro-inflammatory feedback loop.
In addition to ATP, several other neurotransmitters act as messengers between SGCs and neurons. In particular, active neurons release substance P and calcitonin gene-related peptide (CGRP), which, along with ATP, facilitates the activation of the surrounding SGC sheath through paracrine signaling as shown in Fig. 4 (Costa & Neto, 2015). Active SGCs then re-
lease pro-inflammatory cytokines, which, in turn, act on neurons. Substance P is a neuropeptide whose most well-defined function is modulating pain perception by altering cellular signaling pathways (Graefe & Mohiuddin, 2022). This function is consistent with its role in activating SGCs following stimuli. Its receptor is neurokinin type 1 (NK1) as shown in Fig. 4. Furthermore, in an orofacial inflammation model, scientists found that there is both an increase in substance P release as well as NK1 receptor expression (Hanani, 2015). This increase contributes to a rise in cytokine release from SGCs. Another messenger that contributes to SGC activation is CGRP, which is known to be a major factor in the pathophysiology of migraines (Henson et al., 2020). In summary, neurons use ATP, substance P, and CGRP to communicate with and activate SGCs.
As previously mentioned, active SGCs release pro-inflammatory cytokines, including IL-1β and TNF-α as shown in Fig. 4 (Schäfers et al., 2003; Neves et al., 2020). Cytokines recruit leukocytes, including lymphocytes, granulocytes, monocytes, and macrophages, ultimately leading to inflammation around the sensory ganglia (“Cytokine”). The uptake of cytokines by sensory ganglia has also been found to cause hyperalgesia and allodynia in mice, increasing the action potential firing rate in inflamed mice compared to healthy mice (Takeda et al., 2007). These two effects caused by the release of cytokines are believed to contribute to chronic pain.
Since SGCs are non-neuronal cells, they cannot conduct action potentials and are therefore lacking voltage-dependent Na+ and Ca2+ channels (Hanani & Spray, 2020). However, they exhibit Kir4.1 potassium channels which favor intaking K+ into cells over releasing them. With Kir4.1, SGCs can regulate the concentration of intracellular K+ in neurons by manipulating the extracellular K+ concentration, thus changing the concentration gradient between the outside and inside of the neuronal cell membrane. This is significant because the level of intracellular K+ in neurons dictates the resting potential and thus the excitability of the neuron (Costa & Neto, 2015). Thus, mediation of extracellular K+ concentrations by SGC is crucial in pain response. Several studies have demonstrated alterations in SGC Kir4.1 channels (Cherkas et al., 2004). In one mouse model, it was found that there was a significant reduction of K+ currents mediated by Kir4.1 in injured ganglia (Vit et al., 2006). Furthermore, the activation threshold for nociceptors decreased in mice with inflammation compared to healthy mice. Additionally, to further determine if these effects were caused by SGC Kir4.1 channels, scientists downregulated the expression of Kir channels in rats without inflammation. They found that rats began exhibiting pain behaviors even without pain stimuli. Therefore, these studies suggest that the downregulation of Kir4.1 channels contributes to chronic pain and is a characteristic of the SGC pain response mechanism.
Figure 4. Diagram depicting messaging between a neuron and SGC following injury (Adapted from Costa & Neto, 2015)
SGCs are interconnected via gap junctions. Gap junctions are intercellular channels that connect the cytoplasm of two cells, allowing the direct exchange of ions, messengers, and other small molecules between cells (Goodenough & Paul, 2009). Compared with the transmission of messengers across extracellular fluid, gap junctions provide low-resistance pathways that increase the speed of transmission. Gap junctions are expressed in both CNS and PNS glial cells, including SGCs. Gap

junctions found in SGCs are typically connexins (Cx). The most common Cx in mouse DRG and TG are Cx43 and Cx32 (Manteniotis et al., 2013). Cx30.2, Cx37, Cx26, Cx30, Cx45, and Cx36 have also been identified in SGCs (Manteniotis et al., 2013). This is how SGCs connect to form sheaths around neurons. This connection enables the activation of one SGC to spread to other SGCs in the same sheath as shown in Fig. 5a. Cx43 gap junctions do not occur between the cell body of nerves and SGCs.
In animals exhibiting chronic pain, gap junctions appear to connect SGC sheaths of neurons other than the originally activated neuron, enabling signals to be spread to SGCs around adjacent neurons as shown in Fig. 5b. Studies show that this increased gap junction coupling between SGCs occurs following neuropathic injury and results in the hyperexcitability of neurons (Kim et al., 2016). In DRG and TG, Cx43 becomes upregulated following nerve injury or inflammation (Hanani & Spray, 2020). Cx26 expression was also found to have increased in injured TG. Regardless of the type of Cx, evidence indicates that the coupling between SGCs is a vital step in neuropathic pain development and maintenance. In a study on injured spinal ganglia in mice, the mean number of gap junctions for every 100 µm2 of SGC surface was five times greater in injured ganglia than in control ganglia (Pannese et al., 2003), thus demonstrating that new gap junctions form between SGCs following injury. In a dif-
ferent study, Cx43 was inhibited in mice that had chemotherapy-induced chronic neuropathic pain using the drug carbenoxolone (Warwick & Hanani, 2013). Results showed that mice with carbenoxolone exhibited signs of reduced pain, demonstrating that without functional Cx43 coupling, there is reduced pain generation and maintenance.
Figure 5. Dye coupling showing the spread of dye across gap junctions. An asterick marks where the SGC was injected with Lucifer yellow fluorescent dye. a) SGCs are dye-coupled to other SGCs in the sheath. b) In the DRG of a mouse with neuropathic pain, the dye spreads to adjacent SGC sheaths.

(Hanani & Spray, 2020)
A proposed mechanism for how increased gap junction coupling results in neuronal hyperexcitability is that it not only bolsters existing connections between SGCs thus facilitating the spread of pain signals across a single sheath, but it also connects previously separate sheaths, resulting in the spread of pain signals to adjacent sensory neurons (Hanani, 2015). In this model, the pain signal from one neuron spreads to others, resulting in the cross-excitation of sensory ganglia. It is believed that a pain signal leads to ATP, CGRP, and substance P release from the neuron (Hanani & Spray, 2020). As P2 receptors are activated, SGCs are also activated, releasing more ATP and pro-inflammatory cytokines such as TNF-α and IL1β. These cytokines, in turn, attract leukocytes, induce inflammation, and influence the excitability of the neuron through TNF-α and IL1β receptors. The increased concentration of P2 receptors and shift in P2 receptor subtypes further amplifies this response. For sensory ganglions that have increased expression of P2X7R, the effect of ATP is further increased as extracellular calcium diffuses across the gradient. SGCs enable the maintenance of this pain response in chronic pain through sustained Ca2+ waves. The increased gap junction coupling combined with enhanced P2R transmission enables the spread of signals via Ca2+ wave propagation. Calcium activates other SGCs and sensory neurons, reducing extracellular calcium concentration, and increasing ATP release and sensitivity, thus creating a feedback loop that ultimately leads to allodynia and chronic pain. Summarized in Fig 4 and Fig 6, this mechanism relies on abnormal bidirectional neuron-SGC and SGC-SGC communication.
Figure 6. Model of sustained calcium wave across neurons through satellite glial cells and gap junctions (Costa & Neto, 2015)
Given P2X7R’s unique upstream position and its importance in the pain cascade, interruption of it could be a more effective analgesic than current chronic pain treatments. Thus, we present the selective P2X7R antagonist A-740003 to interrupt the ATP release cycle and thus return pathological neuronal behavior to normal.
A-740003 is a competitive antagonist of P2X7R which means that it binds to the same activation site as the agonist (i.e. ATP) but does not activate it, thus blocking the receptor’s function. It has an IC50 value of 40 nM for a human and 18 nM for a rat, while also showing weak to no activity on other P2 receptors, making it a highly selective drug for targeting P2X7 (Honore et al., 2006). Because of its selective properties, the drug can block P2X7 without disrupting the functions of other P2 receptors in the SGC.
Two tests would be performed to analyze the effect of A-740003 on rats with hyperalgesia. The first test would confirm P2X7R activation, blockage, and downstream ef-
fects in vitro, The second test would determine the effect of blockage of P2X7R with A-740003 in live rats exhibiting hyperalgesia.
1) Animals: Experiments would be conducted on live rats in setups according to Neves et al., 2020. Animals would be housed in plastic cages with soft bedding. There would be a 12-hour light/dark cycle with controlled humidity and temperature. Animals would have unlimited access to food and water. All animal handling procedures would be done in accordance with the International Association for the Study of Pain guidelines for the use of animals in pain research in order to ensure that the experiments would be done humanely (“IASP Guidelines for the Use of Animals in Research”).
2) Hyperalgesia Induction Method: Chronic pain often involves hypersensitivity to stimuli that previously would not have yielded as much discomfort, so, to replicate chronic pain, hyperalgesia would be induced by injecting the rat with an inflammatory agent in the sole of the rat’s hind paw, which is part of the L5 DRG (Araldi et al., 2013). 50 µL of Complete Freund’s adjuvant (CFA) would be used as the inflammatory agent, as CFA is a commonly-used antigen that induces autoimmune disease in animal models by enhancing IL‐6 production (Fontes et al., 2017). Mechanical stimuli would then be applied on the same paw of the rat, thus enabling the measurement of hyperalgesia (Neves et al., 2020).
3) A-740003 Drug Delivery Method: In vivo, the P2X7R antagonist A-740003 would be injected following CFA injection. The antagonist would be diluted in a solution of 10% dimethyl sulfoxide (DMSO), 10% propylene glycol, and 80% sterile saline (NaCl 0.9%) as done by Neves et al., 2020. There would be three different doses of differing concentrations, 0.01, 0.10, and 1.00 mM. These concentrations are based on the dose used in an inflammatory pain model by (Neves et al., 2020). The injection technique would be performed by anesthetizing the animal. An ultra-fine needle would then be inserted through the skin

until it hits the L5 DRG (Neves et al., 2020). We would know that the needle has reached the L5 DRG when a paw-flinch reflex is observed (Neves et al., 2020). 5 µL of the solution would then be injected (Neves et al., 2020). The goal of this administration is to isolate the injection to the L5 DRG, ensuring that it does not touch other ganglia such as the spinal cord between the L1-T13 segments (Oliveira et al., 2009).
4) Measurement of Hyperalgesia with Electronic Von Frey Test: Using the electronic Von Frey test, we would be able to measure the level of pain in the rats based on “pain-like” responses to stimuli. Because pain cannot be directly measured in animals, scientists commonly use the Von Frey test. Developed by physiologist Maximilian von Frey in 1894, the test measures pain based on the withdrawal response of the animal following external stimulus (Deuis et al., 2017). In this test, each animal would be placed in a cage with mesh floors, permitting access to the bottom of its paws (Neves et al., 2020). Animals would then be allowed to habituate to the cages until anxiety and exploratory behaviors have calmed down. A filament would then be positioned perpendicularly to the plantar surface of the animal’s hind paw (i.e. same location innervated by the L5 DRG) (Deuis et al., 2017). Then, an increasing force would be applied until the animal voluntarily withdraws its paw. This stimulus would be repeated several times, each time measuring the withdrawal threshold. Animals were tested before and after treatments. To minimize human variation, the same person (blind to the treatments) would conduct all the electronic Von Frey tests (Neves et al., 2020). The results (i.e. the intensity of hyperalgesia) are defined as the difference between the withdrawal threshold before and after treatments.
1) Experimental Setup: To check for P2X7R activation and blockage in SGCs, we would first evaluate changes in intracellular Ca2+ concentration in vitro in a DRG culture. We would measure the Ca2+ concentration before and af-
ter the application of ATP with calcium imaging (Neves et al., 2020). We would also confirm that A-740003 blocks P2X7R by incubating the DRG culture with A-740003 after ATP is added. We would again measure the Ca2+ concentration before and after the application of ATP.
We would also check the downstream effects of P2X7R activation in vitro by stimulating the DRG culture with lipopolysaccharides (LPS) to mimic an injury and induce inflammation. After a given period of time, cultures would then be treated with ATP and A-740003. Finally, an enzyme-linked immunosorbent assay (ELISA) would be used to measure the levels of released cytokines especially TNF-α and IL-1β (Neves et al., 2020).
2) Predicted Results and Discussion: Based on our proposed SGC mechanism, activation of P2X7R should result in increased intracellular calcium concentration in SGCs. Therefore, calcium imaging should show a higher concentration of calcium in SGC sheaths following activation by ATP. In the test where A-740003 is applied, calcium imaging should show little to no change of calcium in SGCs following ATP application. Furthermore, the ELISA should yield high levels of TNF-α and IL-1β in response to injury when no A-740003 is applied in comparison to when A-740003 is applied. If these predicted results are true, it would be confirmed that P2X7R plays a vital role in increasing the intracellular calcium concentration and, thus, facilitating the calcium wave. It would also show that P2X7R activation induces pro-inflammatory cytokines.
F. Blockage of P2X7R with A-740003 in Rats Exhibiting Hyperalgesia in vivo
1) Setup: To determine the effect of blocking P2X7R with A-740003 following the development of peripheral inflammatory hyperalgesia, the inflammatory agent CFA would be first administered into the right hind paw of each animal in this test. Split into four groups, three experimental groups would receive three different doses of A-740003 in the same location (Neves et al., 2020). The control group
would be injected with only saline. After a given time, the electronic von Frey test would then be conducted to measure if A-740003 has an effect on hyperalgesia in the animals.
2) Predicted Results and Discussion: Application of A-740003 should decrease the hyperalgesia compared to the control group. The higher the concentration of A-740003, the greater the tolerance the animals should have to the stimuli. For example, the groups that were administered with 1.00 mM of A-740003 should have a greater tolerance to the stimuli than the 0.10 mM and 0.01 mM groups. The control group should have the least tolerance to the stimuli.
If these predicted results are true, it would demonstrate that the A-740003 blockage of P2X7R reduces hyperalgesia triggered by the inflammatory agent CFA. The likely explanation for this result is that the blockage of P2X7R inhibits the calcium wave and release of cytokines, thus reducing the hyperactivity of the sensory ganglia. Reduction of hyperactivity in neurons could lead to the reduction of chronic pain because nociceptors would return to their normal action potential threshold.
In this paper, we summarized and discussed the known mechanisms and alterations of SGCs in chronic pain. Based on this mechanism, we also proposed a hypothetical experiment of using A-740003 to target P2X7R, a purinergic receptor whose downstream effects of increased calcium uptake and cytokine release contribute to the pathogenesis of the SGC pain response. Further research needs to be done to determine the potential side effects of A-740003, the efficacy of the drug on humans, and if the drug works on other chronic pain models. Other drugs that target the role of SGCs in pain should also be explored. It would be beneficial to further investigate the chemical mechanisms that induce the changes in SGCs described in this paper in order to better develop future drugs. Due to the location of sensory ganglia and SGCs early on in the pain signaling pathway, the close envelopment
of SGCs around neurons, and the lack of the need to directly manipulate neurons, SGCs are a promising therapeutic target for chronic pain.
[1] Araldi, D., Ferrari, L. F., Lotufo, C. M., Vieira, A. S., Athié, M. C. P., Figueiredo, J. G., Duarte, D. B., Tambeli, C. H., Ferreira, S. H., & Parada, C. A. (2013). Peripheral inflammatory hyperalgesia depends on the COX increase in the dorsal root ganglion. Proceedings of the National Academy of Sciences of the United States of America, 110(9), 3603–3608. https://doi.org/10.1073/pnas.1220668110
[2] Blum, E., Procacci, P., Conte, V., & Hanani, M. (2014). Systemic inflammation alters satellite glial cell function and structure. A possible contribution to pain. Neuroscience, 274, 209–217. https://doi.org/10.1016/j.neuroscience.2014.05.029
[3] Cherkas, P. S., Huang, T. Y., Pannicke, T., Tal, M., Reichenbach, A., & Hanani, M. (2004). The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain, 110(1), 290–298. https://doi.org/10.1016/j. pain.2004.04.007
[4] Chessell, I. P., Hatcher, J. P., Bountra, C., Michel, A. D., Hughes, J. P., Green, P., Egerton, J., Murfin, M., Richardson, J., Peck, W. L., Grahames, C. B. A., Casula, M. A., Yiangou, Y., Birch, R., Anand, P., & Buell, G. N. (2005). Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain, 114(3), 386–396. https://doi. org/10.1016/j.pain.2005.01.002
[5] Classification of chronic pain, second edition(Revised). (n.d.). International Association for the Study of Pain (IASP). Retrieved September 1, 2022, from https:// www.iasp-pain.org/publications/free-ebooks/classification-of-chronic-pain-second-edition-revised/ [6] Costa, F. A. L., & Neto, F. L. M. (2015). Satellite glial cells in sensory ganglia: Its role in pain. Brazilian Journal of Anesthesiology (English Edition), 65(1), 73–81. https://doi. org/10.1016/j.bjane.2013.07.013
[7] Cytokine | biochemistry | britannica. (n.d.). Retrieved September 1, 2022, from https://www.britannica.com/science/cytokine
[8] Deuis, J. R., Dvorakova, L. S., & Vetter, I. (2017). Methods used to evaluate pain behaviors in rodents. Frontiers in Molecular Neuroscience, 10, 284. https://doi.org/10.3389/ fnmol.2017.00284
[9] Different types of chronic pain. (2020, March 26). Southern Pain and Neurological. https://southernpainclinic.com/ blog/different-types-of-chronic-pain/
[10] Feldman-Goriachnik, R., Belzer, V., & Hanani, M. (2015). Systemic inflammation activates satellite glial cells in the mouse nodose ganglion and alters their functions. Glia, 63(11), 2121–2132. https://doi.org/10.1002/glia.22881
[11] Fontes, J. A., Barin, J. G., Talor, M. V., Stickel, N., Schaub, J., Rose, N. R., & Čiháková, D. (2017). Complete Freund’s adjuvant induces experimental autoimmune myocarditis by enhancing IL‐6 production during initiation of the immune response. Immunity, Inflammation and Disease, 5(2), 163–176. https://doi.org/10.1002/iid3.155
[12] Freudenrich, C. (2007). How Pain Works. HowStuffWorks. https://science.howstuffworks.com/life/inside-the-
mind/human-brain/pain.htm
[13] Gazerani, P. (2021). Satellite glial cells in pain research: A targeted viewpoint of potential and future directions. Frontiers in Pain Research, 2, 646068. httpsdoi.org/10.3389/ fpain.2021.646068
[14] Goodenough, D. A., & Paul, D. L. (2009). Gap junctions. Cold Spring Harbor Perspectives in Biology, 1(1), a002576. https://doi.org/10.1101/cshperspect.a002576
[15] Graefe, S. B., & Mohiuddin, S. S. (2022). Biochemistry, substance P. In StatPearls. StatPearls Publishing. http:// www.ncbi.nlm.nih.gov/books/NBK554583/
[16] Hanani, M. (2015). Role of satellite glial cells in gastrointestinal pain. Frontiers in Cellular Neuroscience, 9. https:// doi.org/10.3389/fncel.2015.00412
[17] Hanani, M., & Spray, D. C. (2020). Emerging importance of satellite glia in nervous system function and dysfunction. Nature Reviews Neuroscience, 21(9), 485–498. https:// doi.org/10.1038/s41583-020-0333-z
[18] Hanstein, R., Hanani, M., Scemes, E., & Spray, D. C. (2016). Glial pannexin1 contributes to tactile hypersensitivity in a mouse model of orofacial pain. Scientific Reports, 6(1), 38266. https://doi.org/10.1038/srep38266
[19] Henson, B., Hollingsworth, H., Nevois, E., & Herndon, C. (2020). Calcitonin gene-related peptide (Cgrp) antagonists and their use in migraines. Journal of Pain & Palliative Care Pharmacotherapy, 34(1), 22–31. https://doi.org/10.1080/1 5360288.2019.1690616
[20] Honore, P., Donnelly-Roberts, D., Namovic, M. T., Hsieh, G., Zhu, C. Z., Mikusa, J. P., Hernandez, G., Zhong, C., Gauvin, D. M., Chandran, P., Harris, R., Medrano, A. P., Carroll, W., Marsh, K., Sullivan, J. P., Faltynek, C. R., & Jarvis, M. F. (2006). A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. The Journal of Pharmacology and Experimental Therapeutics, 319(3), 1376–1385. https://doi. org/10.1124/jpet.106.111559
[21] IASP guidelines for the use of animals in research. (n.d.). International Association for the Study of Pain (IASP). Retrieved September 1, 2022, from https://www. iasp-pain.org/resources/guidelines/iasp-guidelines-forthe-use-of-animals-in-research/
[22] Inoue, K., Tsuda, M., & Koizumi, S. (2005). ATP receptors in pain sensation: Involvement of spinal microglia and P2X4 receptors. Purinergic Signalling, 1(2), 95–100. https:// doi.org/10.1007/s11302-005-6210-4
[23] Jasmin, L., Vit, J. P., Bhargava, A., & Ohara, P. (2010). Can satellite glial cells be therapeutic targets for pain control? Neuron Glia Biology, 6(1), 63–71. https://doi.org/10.1017/ S1740925X10000098
[24] Kim, Y. S., Anderson, M., Park, K., Zheng, Q., Agarwal, A., Gong, C., Saijilafu, Young, L., He, S., LaVinka, P. C., Zhou, F., Bergles, D., Hanani, M., Guan, Y., Spray, D. C., & Dong, X. (2016). Coupled activation of primary sensory neurons contributes to chronic pain. Neuron, 91(5), 1085–1096. https://doi.org/10.1016/j.neuron.2016.07.044
[25] Kushnir, R., Cherkas, P. S., & Hanani, M. (2011). Peripheral inflammation upregulates P2X receptor expression in satellite glial cells of mouse trigeminal ganglia: A calcium imaging study. Neuropharmacology, 61(4), 739–746. https:// doi.org/10.1016/j.neuropharm.2011.05.019
[26] Manteniotis, S., Lehmann, R., Flegel, C., Vogel, F.,
Hofreuter, A., Schreiner, B. S. P., Altmüller, J., Becker, C., Schöbel, N., Hatt, H., & Gisselmann, G. (2013). Comprehensive rna-seq expression analysis of sensory ganglia with a focus on ion channels and gpcrs in trigeminal ganglia. PLOS ONE, 8(11), e79523. https://doi.org/10.1371/journal. pone.0079523
[27] Neves, A. F., Farias, F. H., de Magalhães, S. F., Araldi, D., Pagliusi, M., Tambeli, C. H., Sartori, C. R., Lotufo, C. M. da C., & Parada, C. A. (2020). Peripheral inflammatory hyperalgesia depends on P2X7 receptors in satellite glial cells. Frontiers in Physiology, 11, 473. https://doi.org/10.3389/ fphys.2020.00473
[28] North, R. A. (2002). Molecular physiology of p2x receptors. Physiological Reviews, 82(4), 1013–1067. https://doi. org/10.1152/physrev.00015.2002
[29] Pannese, E., Ledda, M., Cherkas, P. S., Huang, T. Y., & Hanani, M. (2003). Satellite cell reactions to axon injury of sensory ganglion neurons: Increase in number of gap junctions and formation of bridges connecting previously separate perineuronal sheaths. Anatomy and Embryology, 206(5), 337–347. https://doi.org/10.1007/s00429-002-0301-6 [30] Purinergic receptor—An overview | sciencedirect topics. (n.d.). Retrieved September 1, 2022, from https://www. sciencedirect.com/topics/neuroscience/purinergic-receptor
[31] Purves, D., Augustine, G. J., Fitzpatrick, D., Katz, L. C., LaMantia, A.-S., McNamara, J. O., & Williams, S. M. (2001). Neuroglial cells. Neuroscience. 2nd Edition. https://www. ncbi.nlm.nih.gov/books/NBK10869/
[32] Schäfers, M., Lee, D. H., Brors, D., Yaksh, T. L., & Sorkin, L. S. (2003). Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(7), 3028–3038.
[33] Shinder, V., & Devor, M. (1994). Structural basis of neuron-to-neuron cross-excitation in dorsal root ganglia. Journal of Neurocytology, 23(9), 515–531. https://doi.org/10.1007/ BF01262054
[34] Takeda, M., Tanimoto, T., Kadoi, J., Nasu, M., Takahashi, M., Kitagawa, J., & Matsumoto, S. (2007). Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain, 129(1), 155–166. https://doi.org/10.1016/j.pain.2006.10.007 [35] Vit, J. P., Jasmin, L., Bhargava, A., & Ohara, P. T. (2006). Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biology, 2(4), 247–257. https://doi.org/10.1017/s1740925x07000427
[36] Warwick, R. A., & Hanani, M. (2013). The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. European Journal of Pain (London, England), 17(4), 571–580. https://doi.org/10.1002/j.1532-2149.2012.00219.x
[37] Yam, M., Loh, Y., Tan, C., Adam, S., Manan, N., & Basir, R. (2018). General pathways of pain sensation and the major neurotransmitters involved in pain regulation. International Journal of Molecular Sciences, 19(8), 2164. https://doi. org/10.3390/ijms19082164
[38] Zhang, Y., Laumet, G., Chen, S. R., Hittelman, W. N., & Pan, H. L. (2015). Pannexin-1 up-regulation in the dorsal root ganglion contributes to neuropathic pain development. Journal of Biological Chemistry, 290(23), 14647–14655. https://doi.org/10.1074/jbc.M115.650218
Glioblastoma multiforme (GBM) is one of the most common forms of malignant brain cancer. Despite advancements in technology and treatment over the past century, GBM remains largely incurable. Standard approaches include surgery and combinations of radiotherapy and chemotherapy. This review evaluates the mechanisms and efficacy of standard drugs such as temozolomide and bevacizumab, as well as novel advancements in the field, such as nano-mediated drug delivery systems (NDDS) and the rise of immunology in treating GBM. Factors such as the highly-selective blood-brain barrier have made treating GBM and other brain diseases extremely difficult. However, immunotherapy or “personalized medicine” integrated with chemotherapy or radiotherapy, may become the future for targeting GBM tumors and other brain diseases.
Accounting for more than 78% of brain cancers [1] and causing almost 15,000 deaths every year, glioblastoma multiforme (GBM) is one of the most common and aggressive malignant tumor forms in the central nervous system. GBM is characterized as a high-grade intra-axial tumor because it interferes with brain tissue [1]. Tumors are categorized as “low-grade” or “high-grade” depending on their invasiveness and growth rate [2], with low-grade cancers growing more slowly with less likelihood of metastasizing, or spreading to other sites of the body, than high-grade cancers [1]. GBM develops in glial cells, cells that protect neural tissue, causing a toxic buildup of glutamate, an excitatory neurotransmitter for cell signaling [3]. The excess glutamate kills surrounding neurons, creating brain space for the tumor to expand [4].
A variety of factors are taken into consideration when determining treatment, which may include varying combinations of surgery, radiation, and chemotherapy. Currently only two drugs, temozolomide and bevacizumab, are FDA-approved to treat GBM [5]. Unfortunately, these two chemotherapy drugs have had very limited impact on GBM patient survival rates [6]. Developing alternative and targeted therapies has posed a challenge as glioma tumor cells are protected by the blood-brain barrier (BBB), which is a highly selective semipermeable membrane that acts to protect the brain from pathogens and infections. Due to the barrier’s highly selective permeability, many therapies are unable to cross this boundary [7]. This review will discuss traditional treatments and potentially new technology for the treatment of GBM.
One class of the oldest chemotherapy drugs used for GBM and other cancer forms is alkylating agents, which are able to permeate the BBB [8], making them an optimal choice for GBM treatment. Alkylating agents are used in cancer therapies due to their ability to prevent cells from replicating by inflicting damage to the cell’s DNA [9]. Temozolomide (TMZ) is a typical alkylating agent used for GBM therapy, usually in conjunction with radiotherapy. The drug methylates DNA guanine bases [10], which results in alkylation of the DNA and DNA damage. Subsequently, this triggers apoptosis of malignant cells [11]. However, some tumor cells can become resistant to TMZ’s effects, especially if the tumor cells have mutated and contain the gene MGMT that allows the cancerous cells to repair the DNA damage, preventing apoptosis and continuing the uncontrolled proliferation of the damaged cells [12]. Though TMZ-based chemotherapy demonstrates a comparable improvement in the treatment of patients who have high-grade gliomas, the median increase in survival for patients with GBM is only 2.5 months [13]. Recent studies also indicate that 60-75% of patients with GBM derive no benefit in regards to increased lifespan and quality of life from treatment with TMZ, demonstrating that the drug is only a modestly effective chemotherapy [13]. Additionally, 15-20% of patients who were treated with TMZ developed significant toxicity [14] and side effects such as amnesia and paralysis [15]. While TMZ is a widely-used drug, there is a significant need for chemotherapy or treatment with higher efficacy and safety.
Failure in treating GBM with TMZ chemotherapy leads to the development of monoclonal antibodies and the introduction of targeted immunotherapies. Monoclonal antibodies have been used in therapy processes because of their high affinity for specific proteins involved in brain tumors and other cancers [16]. In 2004, the FDA approved bevacizumab (BEV), which inhibits angiogenesis, the development of new blood vessels, by neutralizing and blocking vascular endothelial growth factor (VEGF), a signaling protein that guides new vessel formation [18]. By targeting tumor growth mechanisms and inhibiting cell growth and division, BEV is able to block oncogenic signaling. Researchers have shown that glioma cells express and secrete VEGF, which has a positive correlation with increased tumor strength and aggressiveness. Since vascular proliferation is a hallmark of glioblastomas, [19], [20], BEV and its VEGF targeting mechanisms have been introduced for GBM. With regards to GBM, bevacizumab slows tumor growth, but it does not cure the actual tumor itself or prolong overall patient survival time [16]. Additionally, rebound phenomena such as tumor recurrence and regrowth are often observed after discontinuation of BEV therapy [21]. Adverse side effects, such as hypertension and proteinuria are also associated with BEV usage [22]. While BEV has been shown to improve the quality of life for patients and has slight efficacy in recurrent GBM [23], [24], it is still only modestly effective in treating GBM overall. With the need for more effective treatment, the basic mechanism of bevacizumab as a monoclonal antibody has led to the development of new immunotherapies and advanced technology systems in treating GBM.
Figure 1. Bevacizumab is a monoclonal antibody that inhibits angiogenesis, the process by which new blood vessels form. It does so by blocking vascular endothelial growth factor (VEGF), a signaling protein that guides new vessel formation and is expressed in glioma cells. It has led to the development of new immunotherapies and advanced alternative treatment options, such as a vaccine targeting VEGF receptors in neurofibromatosis type 2 [62].

Though traditional drugs have had some limited success in treating GBM, nanotherapeutic drug delivery systems (NDDSs) and nanocarriers, transport vehicles for drugs, are rising in popularity as new alternative targeted cancer treatments. Compared to traditional drugs, NDDSs have been shown to have increased advantages when it comes to treating cancers, such as improved stability, enhanced permeability, and highly accurate targeting [25], [26]. Additionally, they have been shown to overcome cancerrelated drug resistance by targeting resistance mechanisms including defective programmed cell death and overexpression of transporters [27]. Using NDDSs for treatment of brain cancer has become a promising alternative, as it is more effective at transporting chemotherapeutics across the BBB than traditional therapies and has minimal side effects on healthy, surrounding tissue [28], [29]. Dp44mt (Di-2-pyridyl ketone-4, 4-di-
methyl-3-thiosemicarbazone) is a novel glioma-targeted nano-therapeutic that has been found to specifically target its toxicity towards glioma cells with no impact on the surrounding healthy tissue [28], [29]. When tested in mice, the Dp44mt nanoparticles reduced tumor growth by 62%. Other chemotherapies, such as TMZ and doxorubicin only reduced tumor growth by 16% [27], [30]–[32]. This may lead to better prognosis, and Dp44mt may serve as a more effective treatment for GBM in humans.
Attached to the nanocarrier, Dp44mt has a gliomatargeted ligand to Interleukin-13 (IL-13), which is found on gliomas [28]. In experimental studies, researchers found that Dp44mt’s conjugation with IL13 receptors on the tumor enhanced glioma cell uptake of the nanocarrier and allowed for more successful permeation of the BBB [28]. Dp44mt is an iron chelator, which extracts excess iron from cells. Though iron is not the underlying cause of many diseases, it does play a role in the rate of disease progression through facilitation of cellular growth and proliferation [33]. For cancer cells, the chelator removes the iron they need for maintaining basic cellular functions, thus starving them [34]. Dp44mt, with the use of a nanocarrier, is the first instance of testing a nano-therapeutic system on brain tumors; it has yielded successful results, as this chelator has been able to overcome multidrug resistance, a common trait of high-grade tumors that renders them immune to chemotherapies [35]. While the drug is still undergoing numerous trials before reaching FDA consideration for approval, certain components of the drug, such as the nanoparticles used to create the carri-
er, have already been approved [29], [36]. Though a novel form of targeted therapy, nanocarriers and nanotherapeutic drug delivery systems hold promise for the future of cancer therapies. However, as this is still a technology undergoing preliminary testing, the drug’s success in animal models may not tranlate completely to patients, and side effects are still unknown in humans. With the uncertainty surrounding this new technology combined with the low efficacy and adverse side effects of traditional treatments,research has found focus on personalized immunotherapy.
Vaccines are among the most standard forms of immunotherapy for bacteria and viruses.Now, vaccines are on the rise to treat diseases such as Alzheimer’s and cancer [37], [38]. Some vaccines that prevent certain viral infections such as human papillomavirus (HPV) and hepatitis B have been modified to serve as cancer vaccines [37]. Due to this repurposing, vaccine therapy for GBM has risen in popularity with the study and development of vaccines in three primary categories: peptide, heat-shock, and cellbased [38]–[41]. Currently, a recent vaccine study for human epidermal growth factor receptor 2 (HER2)-positive breast cancer moved forward after successful results in preventing cancer reformation [42]. In addition to being expressed in breast cancer, upregulated expression of HER2 has been identified in GBM, and could potentially be an immunotherapy target [43]. With the preliminary success of the HER2 vaccine for breast cancer, it could potentially be used as an immunotherapy for GBM as well. Several current Phase I and Phase II trials for GBM studying immunotherapies have shown tumor reduction and lifespan expansion, as 20% of patients in the study survived
from four to five years, which is unusual considering the high fatality of GBM [44]. Compared to other forms of treatment, vaccine immunotherapies are compelling because they have minimal toxicity and can induce a highly patient-personalized anti-tumor response that may be key to eradicating GBM [40]. Additionally, as each vaccine is highly unique to each patient’s immune system, it aligns with the upcoming concept of “personalized medicine” [45], [47]. Personalized medicine is more effective than standard medication as treatment is tailored to the genes of each specific person [45], which has been shown to have high efficacy in cancers such as breast cancer [46], [48]. It may make GBM, one of the most malignant human tumors, manageable for patients while reducing side effects and increasing quality of life [48]. However, vaccine therapy does face some challenges, as surgical removal or biopsy of the tumor may be necessary in order to identify pathology and prepare the vaccine accordingly [49]. Furthermore, because each vaccine is individualized to each patient, this treatment method may not be affordable for all patients. However, as more advances in technology development and existing trials continue, manufacturers may find a cheaper way to create these vaccines. Though it may be an expensive treatment as of now, the precision of personalized medicine can improve the overall quality of life after therapy compared to other treatments, and the results outweigh the cost.
mRNA vaccines have also shown promise in regard to cancer immunotherapy. After vaccination, vehicle-loaded mRNA vaccines express tumor antigens in antigen-presenting cells (APCs), causing APC activation and stimulation of the innate and adaptive immune system [50]. mRNA cancer vaccines hold high promise over other vaccine forms due to their specific toxicity to tumor cells, increased safety, and cost-effectiveness [50], [51]. However, mRNA vaccines have had limitations such as instability in their ability to break down and inefficient delivery in vivo to tumor cells [52]. Nucleotide modifications
and other alterations have been investigated to overcome these challenges, and numerous studies are underway [53]. There also is potential in repurposing treatments, such as the COVID-19 vaccine, to treat GBM. Combinations of mRNA vaccines with other immunotherapies may also increase the anti-tumor immune response. With the recent FDA approval of mRNA vaccines for COVID-19 and promising results of other mRNA cancer vaccines against aggressive solid tumors [51], mRNA vaccines may be a potential immunotherapy treatment for GBM. Though mainstream therapies have had limited success and other forms of immunotherapies are still undergoing trials, the development of chimeric antigen reporter (CAR) T-cell therapy has also shown promise in treating GBM [54]. The treatment relies on using the patients’ collected and genetically engineered cells targeting specific tumor-associated antigens [55]. These cells are harvested from the patient, modified to target particular proteinsthat the tumor expresses, then injected into the patient to destroy the tumor cells [56]. Once the CAR construct binds to the intended target antigen, the T cells are activated, prompting a cytokine release [57]. CAR T has been approved for use in other cancers, such as acute lymphoblastic leukemia and Non-Hodgkin’s lymphoma [59]. Complete remission rates for patients with leukemia undergoing CAR T therapy have been as high as 68%-93%, indicating the treatment’s high efficacy and potential [59], [60]. The approach used in these other cancers is now being applied to treating GBM [59]. There has been evidence that CAR T cells injected directly into the brain tumor tissue or spinal fluid may cause positive responses in patients [60], though a clinical trial is still underway for results to be validated.
The efficacy of CAR T therapy is still yet to be determined in GBM, as only preliminary studies of CAR T in GBM have been conducted. Therefore, it is essential to continue studying CAR T in the context of GBM since prior cancer studies have shown CAR T’s effective-
ness as a treatment option. Its application to GBM is still limited due to the lack of identified tumor-specific antigens expressed in the disease [61]. However, further advances in CAR T, such as multitargeting CAR T therapy, may be effective. Targets such as HER2, IL13, and EGFRvIII have been identified as antigens involved in GBM, but there are numerous other antigens that have yet to be explored [55].
Figure 2. Radiation therapy and chemotherapy have been accepted as traditional forms of treatment for GBM but are still not sufficient. The rise of immunotherapy and “personalized medicine” have led to the development of potential new technology for the treatment of GBM, many of which are undergoing clinical trials and testing.

GBM has been one of the solid tumor cancers that are the most difficult to treat, despite advances in recent technology and medicine. Current standards of care, such as TMZ and radiotherapy, have had limited success in treating patients, often resulting in a myriad of side effects that can be fatal, as well as a significant decrease in the quality of life for patients. As GBM is notoriously difficult to treat due to its high aggressiveness, there is a significant need for treatments with higher efficacy and safety.
Immunotherapy has emerged as a promising choice for treatment, alongside the concept of “personalized medicine.” With numerous treatments under development or undergoing studies and trials, immunotherapies such as vaccines for GBM and CAR T therapy have shown positive results in efficacy, as well as reduced side effects. This review discussed standard forms
of treatment and introduced a new perspective regarding the rise of novel immunotherapies for use in GBM, including vaccines and CAR T. With their revolutionary success in treating other diseases, these therapies have significant potential for GBM. While this review does not have an exhaustive list of therapies, it provides insight into novel therapeutics, building off of the standard treatments currently available.
Based on the direction that these immunotherapies are taking, there is a significant likelihood that future clinical trials will place a greater emphasis on efficacy, safety, immune system mechanisms, and drug resistance prevention. With this, the future of GBM may be combinations of CAR T therapy, vaccines, and other modes of standard treatment, such as chemotherapy, radiation, surgery, etc., making the modern concept of “personalized medicine” a reality.
[1] Wirsching, H. G., Galanis, E., & Weller, M. (2016). Glioblastoma. Handbook of Clinical Neurology, 134. https://doi.org/10.1016/B978-0-12-802997-8.00023-2
[2] Kanderi, T., & Gupta, V. (2021). Glioblastoma Multiforme. In StatPearls [Internet]. StatPearls Publishing.
[3] Siva Kumar Natarajan, S. V. (2019). Glutamine Metabolism in Brain Tumors. Cancers, 11(11). https://doi.org/10.3390/cancers11111628
[4] The Love-Hate Relationship with Glial Cells - Science in the News. (2008, June 16). https://sitn.hms.harvard.edu/flash/2008/issue43/
[5] Jacob P. Fisher, D. C. A. (2021). Current FDAApproved Therapies for High-Grade Malignant Gliomas. Biomedicines, 9(3). https://doi.org/10.3390/biomedicines9030324
[6] Kang, Y. J., Holley, C. K., Abidian, M. R., Madhankumar, A. B., Connor, J., & Majd, S. (2020). Tumor targeted delivery of an anti-cancer therapeutic: An in vitro and in vivo evaluation. Advanced Healthcare Materials, 10(2), 2001261. https://doi.org/10.1002/adhm.202001261
[7] Richard Daneman, A. P. (2015). The Blood–Brain Barrier. Cold Spring Harbor Perspectives in Biology, 7(1). https:// doi.org/10.1101/cshperspect.a020412
[8] Pardridge, W. M. (2012). Drug transport across the
blood–brain barrier. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 32(11), 1959.
[9] Colvin, M. (2003). Alkylating Agents. In Holland-Frei Cancer Medicine. 6th edition. BC Decker.
[10] Zhang, J., Stevens, M. F., & Bradshaw, T. D. (2012). Temozolomide: mechanisms of action, repair and resistance. Current Molecular Pharmacology, 5(1). https://doi. org/10.2174/1874467211205010102
[11] Barciszewska, A. M., Gurda, D., Głodowicz, P., Nowak, S., & Naskręt-Barciszewska, M. Z. (2015). A New Epigenetic Mechanism of Temozolomide Action in Glioma Cells. PloS One, 10(8). https://doi.org/10.1371/journal.pone.0136669
[12] Wesolowski, J. R., Rajdev, P., & Mukherji, S. K. (2010). Temozolomide (Temodar). AJNR. American Journal of Neuroradiology, 31(8), 1383–1384.
[13] Chamberlain, M. C. (2010). Temozolomide: therapeutic limitations in the treatment of adult high-grade gliomas. Expert Review of Neurotherapeutics, 10(10). https://doi.org/10.1586/ern.10.32
[14] Perry, J. R., Bélanger, K., Mason, W. P., Fulton, D., Kavan, P., Easaw, J., Shields, C., Kirby, S., Macdonald, D. R., Eisenstat, D. D., Thiessen, B., Forsyth, P., & Pouliot, J. F. (2010). Phase II trial of continuous doseintense temozolomide in recurrent malignant glioma: RESCUE study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 28(12). https://doi.org/10.1200/ JCO.2009.26.5520
[15] Bae, S. H., Park, M.-J., Lee, M. M., Kim, T. M., Lee, S.-H., Cho, S. Y., Kim, Y.-H., Kim, Y. J., Park, C.-K., & Kim, C.-Y. (2014). Toxicity Profile of Temozolomide in the Treatment of 300 Malignant Glioma Patients in Korea. Journal of Korean Medical Science, 29(7), 980.
[16] Ameratunga, M., Pavlakis, N., Wheeler, H., Grant, R., Simes, J., Khasraw, M., Gynaecological, C., Neurooncology, & Orphan Cancer Group. (2018). Antiangiogenic therapy for high-grade glioma. Cochrane Database of Systematic Reviews , 2018(11). https://doi.org/10.1002/14651858. CD008218.pub4
[17] Iwamoto, F. M., & Fine, H. A. (2010). Bevacizumab for Malignant Gliomas. Archives of Neurology, 67(3), 285–288.
[18] Mukherji, S. K. (2010). Bevacizumab (Avastin). AJNR. American Journal of Neuroradiology, 31(2), 235–236.
[19] Li, Y., Ali, S., Clarke, J., & Cha, S. (2017). Bevacizumab in Recurrent Glioma: Patterns of Treatment Failure and Implications. Brain Tumor Research and Treatment, 5(1), 1.
[20] Ascha, M. S., Wang, J. F., Kumthekar, P., Sloan, A. E.,
Kruchko, C., & Barnholtz-Sloan, J. S. (2019). Bevacizumab for the treatment of non-small cell lung cancer patients with synchronous brain metastases. Scientific Reports, 9(1), 1–9.
[21] Narita, Y. (2013). Drug Review: Safety and Efficacy of Bevacizumab for Glioblastoma and Other Brain Tumors. Japanese Journal of Clinical Oncology, 43(6), 587–595.
[22] Gil-Gil, M. J., Mesia, C., Rey, M., & Bruna, J. (2013). Bevacizumab for the Treatment of Glioblastoma. Clinical Medicine Insights. Oncology, 7, 123.
[23] De Fazio, S., Russo, E., Ammendola, M., Di Paola E, D., & De Sarro, G. (2012). Efficacy and safety of bevacizumab in glioblastomas. Current Medicinal Chemistry, 19(7). https://doi.org/10.2174/092986712799320646
[24] Yu, Z., Zhao, G., Zhang, Z., Li, Y., Chen, Y., Wang, N., Zhao, Z., & Xie, G. (2016). Efficacy and safety of bevacizumab for the treatment of glioblastoma. Experimental and Therapeutic Medicine, 11(2), 371.
[25] Yao, Y., Zhou, Y., Liu, L., Xu, Y., Chen, Q., Wang, Y., Wu, S., Deng, Y., Zhang, J., & Shao, A. (2020). Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Frontiers in Molecular Biosciences, 0. https://doi.org/10.3389/fmolb.2020.00193
[26] Jain, K. K. (2007). Use of nanoparticles for drug delivery in glioblastoma multiforme. Expert Review of Neurotherapeutics, 7(4). https://doi. org/10.1586/14737175.7.4.363
[27] Gallego, L., & Ceña, V. (2020). Nanoparticle-mediated therapeutic compounds delivery to glioblastoma. Expert Opinion on Drug Delivery, 17(11). https://doi.org/10.1080/174 25247.2020.1810015
[28] Holley, C. K., & Majd, S. (2020). Examining the AntiTumor Activity of Dp44mT-Loaded Nanoparticles In Vitro. Conference Proceedings: ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference, 2020. https://doi.org/10.1109/ EMBC44109.2020.9176197
[29] Zhou, J., Jiang, Y., Zhao, J., Zhang, H., Fu, J., Luo, P., Ma, Y., Zou, D., Gao, H., Hu, J., Zhang, Y., & Jing, Z. (2020). Dp44mT, an iron chelator, suppresses growth and induces apoptosis via RORA-mediated NDRG2IL6/JAK2/STAT3 signaling in glioma. Cellular Oncology: The Official Journal of the International Society for Cellular Oncology, 43(3), 461–475.
[30] Alimohammadi, E., Bagheri, S. R., Taheri, S., Dayani, M., & Abdi, A. (2020). The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma multiforme:
a meta-analysis and systematic review. Oncology Reviews, 14(1). https://doi.org/10.4081/oncol.2020.461
[31] Liao, W.-H., Hsiao, M.-Y., Kung, Y., Huang, A. P.-H., & Chen, W.-S. (2021). Investigation of the Therapeutic Effect of Doxorubicin Combined With Focused Shockwave on Glioblastoma. Frontiers in Oncology, 0. https://doi. org/10.3389/fonc.2021.711088
[32] Da Ros, M., Iorio, A. L., De Gregorio, V., Fantappiè, O., Laffi, G., de Martino, M., Pisano, C., Genitori, L., & Sardi, I. (2018). Aldoxorubicin and Temozolomide combination in a xenograft mice model of human glioblastoma. Oncotarget, 9(79), 34935.
[33] Hatcher, H. C., Singh, R. N., Torti, F. M., & Torti, S. V. (2009). Synthetic and natural iron chelators: therapeutic potential and clinical use. Future Medicinal Chemistry, 1(9). https://doi.org/10.4155/fmc.09.121
[34] Cao, L. L., Liu, H., Yue, Z., Liu, L., Pei, L., Gu, J., Wang, H., & Jia, M. (2018). Iron chelation inhibits cancer cell growth and modulates global histone methylation status in colorectal cancer. Biometals: An International Journal on the Role of Metal Ions in Biology, Biochemistry, and Medicine, 31(5). https://doi.org/10.1007/s10534-0180123-5
[35] Holley, C. K., Alkhalifah, S., & Majd, S. (2018). Fabrication and Optimization of Dp44mT-Loaded Nanoparticles. Conference Proceedings: ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference, 2018. https://doi.org/10.1109/ EMBC.2018.8513598
[36] Mirab, F., Kang, Y. J., & Majd, S. (2019). Preparation and characterization of size-controlled glioma spheroids using agarose hydrogel microwells. PloS One, 14(1). https:// doi.org/10.1371/journal.pone.0211078
[37] Mamai, O., Dodagatta-Marri, E., & Akhurst, R. J. (2018). From prevention to cure, repurposing antiviral vaccines for cancer immunotherapy. Biotarget, 2. https:// doi.org/10.21037/biotarget.2018.12.03
[38] Thomas, A. A., Fisher, J. L., Ernstoff, M. S., & Fadul, C. E. (2013). Vaccine-based immunotherapy for glioblastoma. CNS Oncology, 2(4). https://doi.org/10.2217/cns.13.29
[39] Butterfield, L. H. (2016). Lessons learned from cancer vaccine trials and target antigen choice. Cancer Immunology, Immunotherapy: CII, 65(7). https://doi.org/10.1007/s00262-016-1801-1
[40] Xu, L. W., Chow, K. K., Lim, M., & Li, G. (2014). Current vaccine trials in glioblastoma: a review. Journal of Immunology Research, 2014.
https://doi.org/10.1155/2014/796856
[41] Swartz, A. M., Shen, S. H., Salgado, M. A., Congdon, K. L., & Sanchez-Perez, L. (2018). Promising vaccines for treating glioblastoma. Expert Opinion on Biological Therapy, 18(11). https://doi.org/10.1080/14712598.2018.1531846
[42] Knutson, K. L., Block, M. S., Norton, N., Erskine, C. L., Hobday, T. J., Dietz, A. B., & Degnim, A. C. (2020). Rapid Generation of Sustainable HER2-specific T-cell Immunity in Patients with HER2 Breast Cancer using a Degenerate HLA Class II Epitope Vaccine. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 26(5), 1045–1053.
[43] Haynik, D. M., Roma, A. A., & Prayson, R. A. (2007). HER-2/neu Expression in Glioblastoma Multiforme. In Applied Immunohistochemistry & Molecular Morphology (Vol. 15, Issue 1, pp. 56–58). https://doi.org/10.1097/01. pai.0000213133.09160.da
[44] Kong, Z., Wang, Y., & Ma, W. (2018). Vaccination in the immunotherapy of glioblastoma. Human Vaccines & Immunotherapeutics, 14(2), 255.
[45] Li, J., Di, C., Mattox, A. K., Wu, L., & Adamson, D. C. (2010). The future role of personalized medicine in the treatment of glioblastoma multiforme. Pharmacogenomics and Personalized Medicine, 3. https://doi.org/10.2147/ PGPM.S6852
[46] Sabatier, R., Gonçalves, A., & Bertucci, F. (2014). Personalized medicine: present and future of breast cancer management. Critical Reviews in Oncology/hematology, 91(3). https://doi.org/10.1016/j.critrevonc.2014.03.002
[47] Jeibouei, S., Akbari, M. E., Kalbasi, A., Aref, A. R., Ajoudanian, M., Rezvani, A., & Zali, H. (2019). Personalized medicine in breast cancer: pharmacogenomics approaches. Pharmacogenomics and Personalized Medicine, 12, 59.
[48] Taghizadeh, H., Müllauer, L., Furtner, J., Hainfellner, J. A., Marosi, C., Preusser, M., & Prager, G. W. (2019). Applied Precision Cancer Medicine in Neuro-Oncology. Scientific Reports, 9(1), 1–8.
[49] Cuoco, J. A., Benko, M. J., Busch, C. M., Rogers, C. M., Prickett, J. T., & Marvin, E. A. (2018). VaccineBased Immunotherapeutics for the Treatment of Glioblastoma: Advances, Challenges, and Future Perspectives. World Neurosurgery, 120. https://doi.org/10.1016/j.wneu.2018.08.202
[50] Miao, L., Zhang, Y., & Huang, L. (2021). mRNA vaccine for cancer immunotherapy. Molecular Cancer, 20(1). https://doi.org/10.1186/s12943-021-01335-5
[51] Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines — a new era in vaccinology. Nature Reviews. Drug Discovery, 17(4), 261.
[52] Tang, X., Zhang, S., Fu, R., Zhang, L., Huang, K., Peng, H., Dai, L., & Chen, Q. (2019). Therapeutic Prospects of mRNA-Based Gene Therapy for Glioblastoma. Frontiers in Oncology, 9. https://doi.org/10.3389/fonc.2019.01208 [53] Wang, Y., Zhang, Z., Luo, J., Han, X., Wei, Y., & Wei, X. (2021). mRNA vaccine: a potential therapeutic strategy. Molecular Cancer, 20(1), 1–23.
[54] Bagley, S. J., Desai, A. S., Linette, G. P., June, C. H., & O’Rourke, D. M. (2018). CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro-Oncology, 20(11). https://doi.org/10.1093/neuonc/noy032 [55] Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021). CAR T Cell-Based Immunotherapy for the Treatment of Glioblastoma. Frontiers in Neuroscience, 0. https://doi.org/10.3389/fnins.2021.662064
[56] Waldman, A. D., Fritz, J. M., & Lenardo, M. J. (n.d.). A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nature Reviews. Immunology, 1. [57] Miliotou, A. N., & Papadopoulou, L. C. (2018). CART cell Therapy: A New Era in Cancer Immunotherapy. Current Pharmaceutical Biotechnology, 19(1). https://doi.org/10 .2174/1389201019666180418095526
[58] Bupha-Intr, O., Haeusler, G., Chee, L., Thursky, K., Slavin, M., & Teh, B. (2021). CAR-T cell therapy and infection: a review. Expert Review of Anti-Infective Therapy, 19(6). https://doi.org/10.1080/14787210.2021.1855143
[59] Land, C. A., Musich, P. R., Haydar, D., Krenciute, G., & Xie, Q. (2020). Chimeric antigen receptor T-cell therapy in glioblastoma: charging the T cells to fight. Journal of Translational Medicine, 18(1), 1–13.
[60] Akhavan, D., Alizadeh, D., Wang, D., Weist, M. R., Shepphird, J. K., & Brown, C. E. (2019). CAR T cells for brain tumors: Lessons learned and road ahead. Immunological Reviews, 290(1). https://doi.org/10.1111/imr.12773
[61] Karschnia, P., Teske, N., Thon, N., Subklewe, M., Tonn, J.-C., Dietrich, J., & von Baumgarten, L. (2021). Chimeric Antigen Receptor T Cells for Glioblastoma. Neurology, 97(5), 218–230.
[62] Tamura, R., Fujioka, M., Morimoto, Y. et al. A VEGF receptor vaccine demonstrates preliminary efficacy in neurofibromatosis type 2. Nat Commun 10, 5758 (2019). https://doi.org/10.1038/s41467-019-13640-1
This summer, I had the opportunity to intern at the Coriell Institute for Medical Research for the Coriell Summer Experience Program located in Camden, New Jersey. The Coriell Institute operates as a bio-bank and research facility with a mission to “accelerate scientific discovery by generating world-class biomaterials and conducting groundbreaking research in biobanking, personalized medicine and stem cell biology”.
As an intern in the Stem Cell Lab, I learned the methods to preserve induced pluripotent stem cells using sterile technique. With the guidance of my mentors, I was able to maintain my own stem cell line: feeding, splitting, and aspirating the cells. Aside from wet lab practice, I learned the equal importance of photography in the lab. After each split, feed, or aspiration, I took pictures of the cells under the microscope. Under the microscope, I could understand if the cells were differentiating and if I would need to split the cells to prevent continual differentiation. As the culminating project, my co-intern and I delivered a formal presentation to the CEO and Coriell staff. My summer at Coriell exceeded all of my expectations for the program. Every single staff member made each intern feel welcomed and appreciated. I would recommend the internship opportunity to anyone interested in pursuing a career in the scientific field.
*An EB Harvest of my stem cell line
Email Mirika Jambudi (VI): mjambudi2023@pingry.org

Evan Xie (V): exie2024@pingry.org
Annabelle Shilling (V): ashilling2022@pingry.org
Mr. Maxwell: dmaxwell@pingry.org

