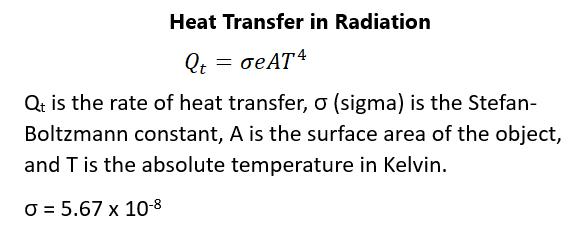
molecular motion would theoretically stop. In physics and many scientific circles, it’s the Kelvin scale that is used. For the calculation from Fahrenheit to Celsius, you take nine-fifths of the difference between the Fahrenheit degrees and 32. The difference between Kelvin and Celsius is 273.15 degrees, with the Celsius scale just being a version of the Kelvin scale. Standard temperature is said to be 25 degrees Celsius, which is approximately “room temperature”. You should know that absolute zero on the Kelvin scale isn’t the lowest possible temperature recorded or possible. The coldest temperature on Earth recorded has been 183 degrees Kelvin or -89 degrees Celsius. The lowest temperature recorded has been 1 x 10-10 degrees Kelvin, which is far below the level of absolute zero. The coldest place outside Earth in the known universe outside of a laboratory is 1-degree Kelvin in the Boomerang Nebula. The highest known temperature in experimental science is in the range of 1012 degrees Kelvin. In the known universe, the temperature of the interior of a neutron star is 109 degrees Kelvin. You need to recognize that thermometers take their own temperature and not necessarily the temperature of their environment. There needs to be thermal contact between the thing being measured and the thermometer so that there is thermal equilibrium. Time must pass before this can truly happen as there needs to be transfer of heat. If two systems are in thermal equilibrium and a third system is in equilibrium with a third system, all three systems are in equilibrium. The Zeroth law of thermodynamics indicates that this is true.
KINETIC THEORY Kinetic theory is related to all phases, gaseous, liquid, and solid; however, it is most applied to gases. This is because the pressure of gases is highly related to the kinetics of different gas molecules at various temperatures. The assumption with gases is that atoms and molecules are in continuous random motion dependent on temperature. Pressure of gases can be explained by kinetic theory. In a chamber of N numbers of gas molecules with a single molecule mass of m in a certain volume V will collide with one 153