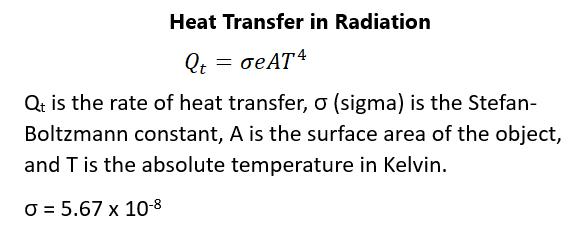
the internal energy is just three-halves times N times Boltzmann constant times the temperature. What this means is that the degrees of freedom matter when it comes to the internal energy of a gas. Each degree of freedom contributes one-half times the Boltzmann constant times Temperature. So, what is Boltzmann constant? Without getting into the specifics of how this is calculated, you need to know that this is the gas constant R divided by Avogadro’s number (which is the number of atoms in a mole of a substance). Boltzmann constant equals 1.38 × 10-23 kilogram-meters squared per second squared per degree Kelvin or Joules per degree Kelvin. The average kinetic energy of a molecule is independent of the type of molecule. The average translational kinetic energy depends only on the absolute temperature (multiplied by a constant). This kinetic energy is very small when you think of its macroscopic energies so that one does not feel the collision of air molecules on the skin, just the total macroscopic pressure. The root-mean-square velocity of a molecule is the square root of the sums of the velocities squared of the velocities in all three axes. The mean free path, which is the distance a molecule can move on average between collisions of molecules, is very small. The faster the root mean velocity of air molecules, the faster that sound vibrations can be transferred through the air. The speed of sound increases with the temperature and is greater in gases that have smaller molecules. This is why people sound differently when inhaling helium.
THERMAL EXPANSION OF LIQUIDS AND SOLIDS Thermal expansion of gases is obvious but you need to know that solids and liquids expand with greater temperature. Raising the temperature of the gas in a balloon will increase its buoyant force or upward force. Alcohol will expand in an alcohol thermometer. Bridges and railroad tracks will have expansion joints that allow them to expand freely in higher temperatures and contract freely in lower temperatures. Thermal expansion depends on the substance as well as the actual temperature.
157