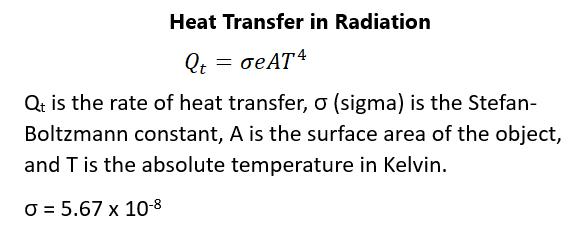
PHASE CHANGES Within the range of temperatures, a substance can be in gaseous, liquid, or solid form. Gases act very much like ideal gases at high temperatures but fail at lower temperatures. Molecules will interact with one another and condensation occurs. There will be a significant decrease in volume as the substance becomes liquid. At even lower temperatures, the substance becomes solid. The volume becomes smaller but does not reach zero due to the mass of the molecules. Gases will become liquid at high pressures as well. Pressure-volume diagrams will plot the behavior of a substance based on these parameters. When a substance is an ideal gas, there is a particular relationship between pressure and volume. The volume of a gas will decrease as the pressure increases. At fixed temperatures, this leads to a series of hyperbolas called isotherms. At some point, the temperature becomes too low and the gas no longer behaves ideally. There is a critical point above which a liquid cannot exist. The critical pressure is the minimum pressure needed for liquid to exist at a critical temperature. Figure 76 shows these values:
Figure 76.
161