the liquid phase. Other substances, like the phenol in moth balls, will go through sublimation. All phase changes will involve heat transfer. In the case of a solid to vapor transition, the energy required is mass times the latent heat of sublimation, which is the energy required to change a kilogram of a substance to vapor via sublimation. This value is a different value than the latent heats of fusion and the latent heats of vaporization and entirely depend on the substance. As in all phase changes, there is heat necessary to have sublimation occur.
HEAT TRANSFER METHODS In this section, the different methods of heat transfer are introduced. Whenever there is a temperature difference, heat transfer will occur. It can be rapid or slow and can occur through different routes. There are things that will control heat transfer, such as wearing layers to control heat loss, using insulation, and having white roofs to reflect the summer sunlight. So many processes in real-life circumstances will involve heat transfer so that it is difficult to imagine a situation where there is absolutely no heat transfer. The three main mechanisms of heat transfer to be discussed include these mechanisms: •
Conduction—this is heat transfer in stationary matter that occurs by physical contact. The term “stationary” refers to the macroscopic object. Molecularly, there is continual thermal motion of atoms and molecules at any temperature above absolute zero. An example of this is the heat transfer from an electric stove burner and the bottom of a pan.
•
Convection—this is heat transfer by the macroscopic movement of a fluid and is the type of transfer that takes place in forced-air furnaces and in the weather itself.
•
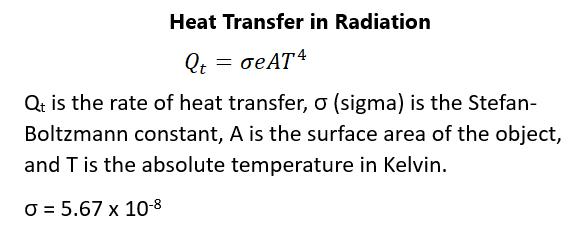
Radiation—this is the type of heat transfer that happens when the sun warms the earth, when microwaves heat food, and even when there is thermal radiation emanating from the human body.
174