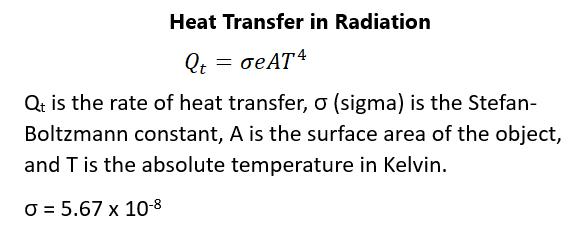
CONDUCTION Conduction can be experienced in several everyday ways. The cold in a house is felt more acutely on bare floors than it is on carpeting, even though the temperature of both are the same. This is because some materials will conduct thermal energy faster than others. In general, things that conduct electricity well are also good heat conductors, while things that don’t conduct electricity are poor heat conductors. The average kinetic energy of any molecule in a hot object will be higher than the average kinetic energy of a cold object. If two molecules collide, the energy from the molecule with a greater kinetic energy will be transferred to the molecule with a lesser kinetic energy. This will result in a net flux of heat from the hot object to a cold object. The heat flux will depend on the temperature difference of the two objects. Eventually the heat transfer decreases to zero and equilibrium is achieved. What is obvious is that the conduction of heat depends on the cross-sectional area between the two objects. The greater the cross-sectional area, the faster the rate of heat transfer. Another factor in conduction is the thickness of the material through which the heat transfers. The thicker the material, the longer it takes to transfer the energy. It explains why thick clothing is more protective against the cold than thin clothing. The final thing that affects the rate of heat transfer is a coefficient of thermal conductivity, which is dependent upon the substance. This leads to the equation for the rate of heat transfer, which is shown in figure 80:
175