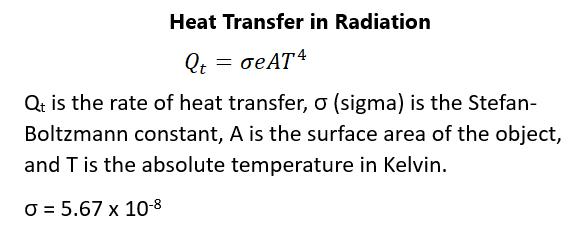
RADIATION Radiation happens whenever heat comes from a fire or from an energy source like a microwave or the Sun. Similarly, you can feel the heat from an oven even without touching it. No conduction and no convection happen when the sun shines on the earth because there is no contact and no air movement. There is no medium required for the propagation of electromagnetic waves. These waves can come from x-rays, gamma rays, microwaves, infrared radiation, radio waves, visible light, and ultraviolet radiation. With the heat transfer associated with fires, it isn’t the visible light that causes heat transfer but the infrared radiation. Visible light actually transmits little thermal energy. Convection is in play as it transfers energy away from the observers because of wind and rising air secondary to decreased density. As you can imagine, conduction plays little role in heat transfer in the case of fires. What’s true is that all objects absorb and give off electromagnetic radiation in some form or another. The rate of heat transfer depends on the color of the object. Black will absorb heat more readily and white will reflect it to a greater degree. Black also radiates heat better than lighter objects. The ideal radiator is therefore the ideal absorber, while poor absorbers will radiate heat more poorly. Colored objects will behave differently, depending on where they are in the visible range. Skin, for example, absorbs infrared radiation more readily and makes people more sensitive to this type of radiation. The rate of heat transfer by means of emitted radiation can be determined by what’s called the Stefan-Boltzmann law of radiation. This relates to the rate of heat transfer rather than the actual heat transferred. Figure 81 shows the equation:
178