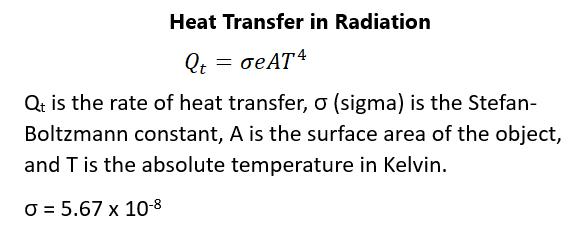
QUIZ 1. What is the best physics definition of heat? a. It is the internal energy of a system. b. It is the work done between two systems of different energy levels. c. It is the potential of the system to do work because of its internal energy. d. It is the spontaneous transfer of internal energy from one object to another. Answer: d. Heat is not the same thing as temperature. It involves the spontaneous transfer of stored internal energy from one object to another, which is transferred because of differences in temperature. 2. What is the SI unit for heat? a. Joule b. Degree c. Kilocalorie d. Calorie Answer: a. Remember that heat is energy and, in all cases, SI units for energy are joules, regardless of the type of energy being talked about. Degrees have nothing to do with actual energy and both the calorie and kilocalorie are non-SI units for heat energy. 3. On what condition or situation is heat transfer least dependent upon? a. Mass of the system b. Temperature change in the system c. Phase of the objects d. Potential energy of the system Answer: d. The mass of the system, the phase of the objects, and the change in temperature of the system are highly linked to heat transfer; however, the potential energy of the system is not usually related to heat transfer.
181