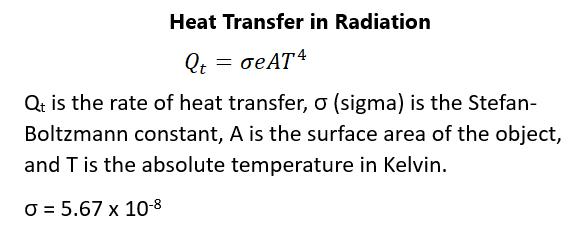
Note that figure 83 is a perfect system in which every process is reversible when, in fact, many thermodynamic processes are not reversible and involve a loss of heat from the system. Later on, we will see a more realistic picture of what this looks like in a fourstroke engine. In some work situations involving pressure and volume, there can be an adiabatic process, which is defined as a process that has no transfer of heat. This means that Q equals zero. These can only be done if there is a great deal of insulation in the system or if the process is so fast that heat transfer does not occur. In fact, in an adiabatic process, the change in temperature must be negative because work will be done at the expense of internal energy. An example of an adiabatic process is the release of gas into the air by a pressurized cylinder. The gas will be colder than the gas in the cylinder and there will be less work done. Figure 84 describes these simple engines and the processes involved:
Figure 84.
SECOND LAW OF THERMODYNAMICS While, in theory, work can be done that is reversible, this doesn’t take place in most thermodynamic processes. In theory, an irreversible process is one that will depend on the path taken. A cold object next to a hot object will never get colder because the direction in heat only goes in one direction—from hot to cold. Friction is irreversible as well because, when it stops an object, it does not cool the object so it moves again. Gases
189