KEY TAKEAWAYS •
The first law of thermodynamics states that the change in internal energy of a system is the difference between the heat put in and the work output of the system.
•
Heat energy into the system is positive and work energy out of the system is positive.
•
There are adiabatic, isothermal, isochoric, and isobaric processes involved in energy systems.
•
The work done is the area bound by a pressure-volume curve in an engine.
•
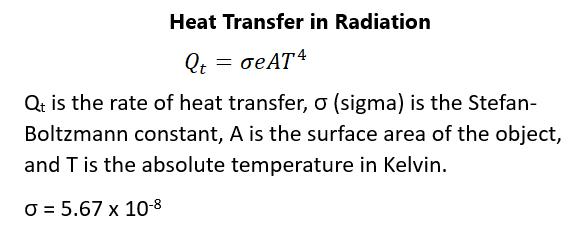
Heat always goes from hot to cold areas in a system.
•
A Carnot engine involves a theoretical engine in which the processes are always reversible.
•
The second law of thermodynamics applies also to the fact that entropy is always increasing or held constant.
•
In order to decrease the entropy of a system, energy must be put into it from an external source.
201