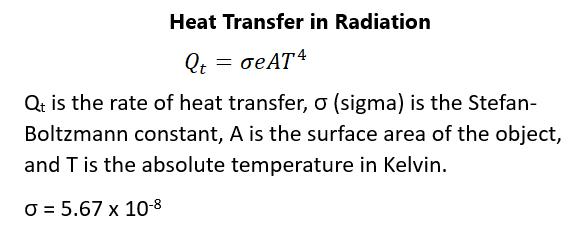
ELECTRICAL FIELDS Any type of contact force, such as between a tennis racquet and ball, can be explained through interactions of forces of atoms and molecules that are in close proximity to one another. The force that explains this is Coulomb force. This must be contact force because the action of charges between molecules occurs over extremely close distances. All objects are surrounded by a force field, however, small, that has the ability to act on another object a short distance away. This is how charged objects can act on other objects over a distance. In this way, the Coulomb force field is the force field around any charged object in space. Coulomb’s law can be rewritten to be the force being proportional to a point charge, indicated by the capital letter Q, acting on a test charge, indicated by the small letter q, over the distance between them squared. Figure 108 describes this:
Figure 108.
Coulomb’s force fields will still involve repulsive forces with like charges and attractive forces on unlike charges. The magnitude of the force will be greater if the charge on either one of the objects is greater. What this means is that the magnitude will be proportional to the degree of charge on either side. The force field is not unique to any point in space but instead depends on the charge magnitude of either object. If the test charge is changed and the point charge is held the same, the electric field is the total force (the Coulomb force) divided by the test charge q. The electric field E will
231